
Contributions
Abstract: S241
Type: Oral Presentation
Session title: Stem cell transplantation - Clinical
Background
Transplant-associated thrombotic microangiopathy (TA-TMA) is a serious complication of hematopoietic stem cell transplantation that is associated with significant mortality and morbidity and for which there is currently no approved therapy. TA-TMA results from endothelial injury caused by conditioning regimens, immunosuppressants, infection, and GVHD, which in turn activates the lectin pathway of complement.
Aims
Narsoplimab, an inhibitor of mannan-binding lectin-associated serine protease-2 (MASP-2), the effector enzyme of the lectin pathway and an activator of the coagulation cascade, was studied for treatment of TA-TMA.
Methods
This was a single-arm open-label pivotal trial in adult TA-TMA patients (NCT02222545). All patients provided informed consent. Protocol-specified treatment consisted of IV narsoplimab 4 mg/kg or 370 mg once weekly for 4 or 8 weeks with a 6-week follow-up period. The FDA-agreed primary endpoint required clinical improvements in each of 2 categories: 1) laboratory markers of TMA (platelet count and LDH) and 2) organ function (kidney, pulmonary, gastrointestinal, or neurological) or freedom from transfusion (platelets and/or red blood cells). Secondary endpoints included change from baseline in platelet count, LDH, hemoglobin, and haptoglobin. Patients receiving at least 1 dose of narsoplimab (full analysis set [FAS]; N=28) and patients receiving the protocol-specified narsoplimab treatment of at least 4 once-weekly doses (per-protocol [PP]; N=23) were analyzed.
Results
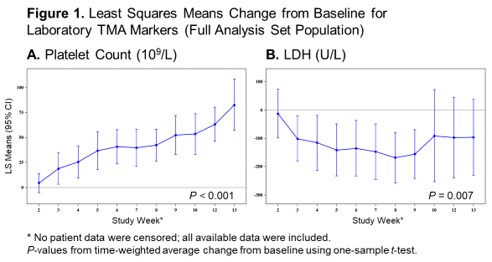
Patients were at high risk for poor outcomes and had multiple comorbidities: kidney and neurological dysfunction were present in 75% (21/28) and 57% (16/28) of patients, respectively, at baseline. Patients received a range of 2–8 scheduled doses of narsoplimab (mean 6.3 doses) and median duration of treatment was 8 weeks. Narsoplimab treatment resulted in clinical response (achievement of the primary endpoint) in 61% (17/28) of the FAS and in 74% (17/23) of the PP population, based on improvements in both laboratory TMA markers and clinical status (organ function and transfusion burden). The time to hematological response (defined as time from first narsoplimab dose to time of achieving both platelet count and LDH primary endpoint criteria) was as early as 7 days (median 36 days) after start of treatment. There was a sustained improvement in platelet count and LDH change from baseline (Figure 1). Five patients never achieved platelet engraftment following transplant and were not included in the platelet count analysis. Improvements in kidney function, neurological function, or gastrointestinal function were observed in 67% (18/27), 50% (3/6), and 100% (1/1), respectively, in the FAS and 68% (15/22), 50% (3/6), and 100% (1/1), respectively, in the PP. No patients were evaluable for improvement in pulmonary function. Of patients who received transfusions at baseline, 48% (12/25) in the FAS and 55% (11/20) in the PP achieved freedom from platelet and red blood cell transfusion. Six patients died during the core study period: 1 of septic shock, 2 of progressive AML, 2 of neutropenic sepsis, and 1 of GVHD and TMA. These deaths occurred 3–42 days following the last narsoplimab dose.
Conclusion
In this high-risk patient population with TA-TMA, there were no safety concerns observed with narsoplimab, and treatment resulted in clinically meaningful improvements in laboratory markers of TMA and in organ function or freedom from transfusion.
Keyword(s): Complement, Stem cell transplant, Thrombotic microangiopathy, Treatment
Abstract: S241
Type: Oral Presentation
Session title: Stem cell transplantation - Clinical
Background
Transplant-associated thrombotic microangiopathy (TA-TMA) is a serious complication of hematopoietic stem cell transplantation that is associated with significant mortality and morbidity and for which there is currently no approved therapy. TA-TMA results from endothelial injury caused by conditioning regimens, immunosuppressants, infection, and GVHD, which in turn activates the lectin pathway of complement.
Aims
Narsoplimab, an inhibitor of mannan-binding lectin-associated serine protease-2 (MASP-2), the effector enzyme of the lectin pathway and an activator of the coagulation cascade, was studied for treatment of TA-TMA.
Methods
This was a single-arm open-label pivotal trial in adult TA-TMA patients (NCT02222545). All patients provided informed consent. Protocol-specified treatment consisted of IV narsoplimab 4 mg/kg or 370 mg once weekly for 4 or 8 weeks with a 6-week follow-up period. The FDA-agreed primary endpoint required clinical improvements in each of 2 categories: 1) laboratory markers of TMA (platelet count and LDH) and 2) organ function (kidney, pulmonary, gastrointestinal, or neurological) or freedom from transfusion (platelets and/or red blood cells). Secondary endpoints included change from baseline in platelet count, LDH, hemoglobin, and haptoglobin. Patients receiving at least 1 dose of narsoplimab (full analysis set [FAS]; N=28) and patients receiving the protocol-specified narsoplimab treatment of at least 4 once-weekly doses (per-protocol [PP]; N=23) were analyzed.
Results
Patients were at high risk for poor outcomes and had multiple comorbidities: kidney and neurological dysfunction were present in 75% (21/28) and 57% (16/28) of patients, respectively, at baseline. Patients received a range of 2–8 scheduled doses of narsoplimab (mean 6.3 doses) and median duration of treatment was 8 weeks. Narsoplimab treatment resulted in clinical response (achievement of the primary endpoint) in 61% (17/28) of the FAS and in 74% (17/23) of the PP population, based on improvements in both laboratory TMA markers and clinical status (organ function and transfusion burden). The time to hematological response (defined as time from first narsoplimab dose to time of achieving both platelet count and LDH primary endpoint criteria) was as early as 7 days (median 36 days) after start of treatment. There was a sustained improvement in platelet count and LDH change from baseline (Figure 1). Five patients never achieved platelet engraftment following transplant and were not included in the platelet count analysis. Improvements in kidney function, neurological function, or gastrointestinal function were observed in 67% (18/27), 50% (3/6), and 100% (1/1), respectively, in the FAS and 68% (15/22), 50% (3/6), and 100% (1/1), respectively, in the PP. No patients were evaluable for improvement in pulmonary function. Of patients who received transfusions at baseline, 48% (12/25) in the FAS and 55% (11/20) in the PP achieved freedom from platelet and red blood cell transfusion. Six patients died during the core study period: 1 of septic shock, 2 of progressive AML, 2 of neutropenic sepsis, and 1 of GVHD and TMA. These deaths occurred 3–42 days following the last narsoplimab dose.

Conclusion
In this high-risk patient population with TA-TMA, there were no safety concerns observed with narsoplimab, and treatment resulted in clinically meaningful improvements in laboratory markers of TMA and in organ function or freedom from transfusion.
Keyword(s): Complement, Stem cell transplant, Thrombotic microangiopathy, Treatment


