
Contributions
Abstract: EP994
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
In MM treatment, it is important to improve our understanding of routine clinical practice and the effectiveness of new agents outside of clinical trials. Ixazomib (Ixa) is approved for MM patients (pts) who have received ≥1 prior therapy on the basis of TOURMALINE-MM1 study data.
Aims
Report data from the final analysis of UVEA-IXA, a European, multicenter, observational, longitudinal cohort study of pts receiving Ixa via an Early Access Program (EAP) in the Czech Republic, Greece, Hungary, Italy, Slovakia, Slovenia, Spain, and United Kingdom.
Methods
UVEA-IXA comprised a retrospective chart review from Ixa therapy initiation in the EAP and a prospective 1-year (yr) follow-up period from chart review initiation, with quarterly data capture. Eligible pts were in biochemical and/or symptomatic relapse after 1–3 prior lines of therapy, had not received anti-MM therapy for >3 cycles (except steroids) at the start of Ixa therapy, and had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2. Lenalidomide (R)- or proteasome inhibitor-refractory pts were ineligible. Primary endpoints were response and progression-free survival (PFS).
Results
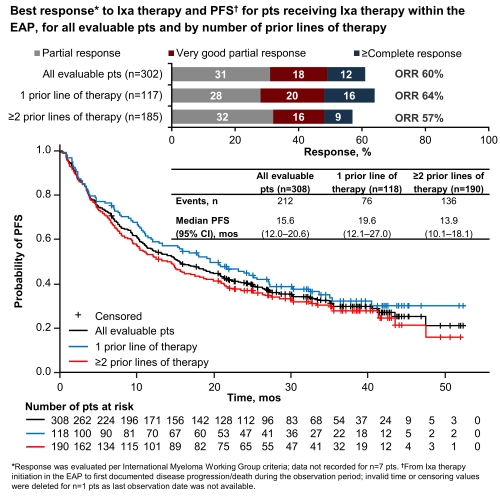
309/357 enrolled pts were evaluable; 54% were male; median age at enrollment was 68 yrs (range 36–92), 40% aged ≥70 yrs. At the start of Ixa therapy, 37/123 pts (30%; n=186 unknown [unk]) had International Staging System (ISS) stage III MM, 62/308 (20%; n=1 unk) had ECOG PS 2, and 13/279 (5%; n=30 unk) had an estimated glomerular filtration rate (eGFR) <30 mL/min. Of 309 pts, 61% had ≥1 comorbidity, including hypertension (26%), renal disease (23%), and diabetes (9%). Median time from diagnosis was 37.2 months (mos; range 4.9–232.8); 39%, 43%, and 18% of pts had received 1, 2, and 3 prior lines of therapy, respectively. Median observation period (n=306; n=3 not available) was 25.5 mos (range 0.8–54.1). Pts received Ixa for a median of 10.5 mos (range 0.2–52.3); most pts also received R (98%) and dexamethasone (97%). Overall response rate (ORR) on Ixa therapy was 60%; median PFS (mPFS) was 15.6 mos (Figure). Risk of progression/death was significantly greater in pts with ECOG 2 vs 0–1: 87% vs 64% had PFS events (p=0.0004). Overall, median time to next therapy after stopping Ixa was 21.4 mos. Median overall survival was 35.5 mos (95% confidence interval [CI] 28.0–44.4) overall, and 43.1 vs 31.4 mos (95% CI 31.2–not evaluable vs 21.5–47.5) in pts with 1 vs ≥2 prior lines of therapy. Ixa dose was reduced in 57/307 pts (19%; n=2 not recorded); 246/309 pts (80%) discontinued Ixa, most frequently due to: progression in 37% of pts, adverse events (AEs) in 19%, and loss/lack of response in 10%. Among 215 pts who received ≥4 cycles of Ixa, rates of any‑grade (G) and G≥3 AEs were 62% and 37%; most common any-G AEs were thrombocytopenia (15%) and diarrhea (13%); the most common G≥3 AE was thrombocytopenia (6%).
Conclusion
UVEA-IXA data show that Ixa therapy is an effective, tolerable treatment option outside the clinical trial setting. Outcomes were favorable in pts with 1 vs ≥2 prior lines of therapy and ECOG PS 0–1 vs 2. Although UVEA-IXA data derive from retrospective chart review/infrequent prospective data capture and thus cannot be directly compared to clinical trial data, compared with TOURMALINE‑MM1 pts (Ixa arm; ORR 78%, mPFS 20.6 mos), UVEA-IXA pts had higher rates of ECOG PS 2 (20 vs 5%), ISS stage III MM (30 vs 12%), and eGFR <30 mL/min (5 vs 1%), and had received more prior lines of therapy (61 vs 38% had ≥2). Most common AEs were gastrointestinal and hematologic, consistent with the safety profile in TOURMALINE-MM1.
Keyword(s): Multiple myeloma, Proteasome inhibitor
Abstract: EP994
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
In MM treatment, it is important to improve our understanding of routine clinical practice and the effectiveness of new agents outside of clinical trials. Ixazomib (Ixa) is approved for MM patients (pts) who have received ≥1 prior therapy on the basis of TOURMALINE-MM1 study data.
Aims
Report data from the final analysis of UVEA-IXA, a European, multicenter, observational, longitudinal cohort study of pts receiving Ixa via an Early Access Program (EAP) in the Czech Republic, Greece, Hungary, Italy, Slovakia, Slovenia, Spain, and United Kingdom.
Methods
UVEA-IXA comprised a retrospective chart review from Ixa therapy initiation in the EAP and a prospective 1-year (yr) follow-up period from chart review initiation, with quarterly data capture. Eligible pts were in biochemical and/or symptomatic relapse after 1–3 prior lines of therapy, had not received anti-MM therapy for >3 cycles (except steroids) at the start of Ixa therapy, and had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2. Lenalidomide (R)- or proteasome inhibitor-refractory pts were ineligible. Primary endpoints were response and progression-free survival (PFS).
Results
309/357 enrolled pts were evaluable; 54% were male; median age at enrollment was 68 yrs (range 36–92), 40% aged ≥70 yrs. At the start of Ixa therapy, 37/123 pts (30%; n=186 unknown [unk]) had International Staging System (ISS) stage III MM, 62/308 (20%; n=1 unk) had ECOG PS 2, and 13/279 (5%; n=30 unk) had an estimated glomerular filtration rate (eGFR) <30 mL/min. Of 309 pts, 61% had ≥1 comorbidity, including hypertension (26%), renal disease (23%), and diabetes (9%). Median time from diagnosis was 37.2 months (mos; range 4.9–232.8); 39%, 43%, and 18% of pts had received 1, 2, and 3 prior lines of therapy, respectively. Median observation period (n=306; n=3 not available) was 25.5 mos (range 0.8–54.1). Pts received Ixa for a median of 10.5 mos (range 0.2–52.3); most pts also received R (98%) and dexamethasone (97%). Overall response rate (ORR) on Ixa therapy was 60%; median PFS (mPFS) was 15.6 mos (Figure). Risk of progression/death was significantly greater in pts with ECOG 2 vs 0–1: 87% vs 64% had PFS events (p=0.0004). Overall, median time to next therapy after stopping Ixa was 21.4 mos. Median overall survival was 35.5 mos (95% confidence interval [CI] 28.0–44.4) overall, and 43.1 vs 31.4 mos (95% CI 31.2–not evaluable vs 21.5–47.5) in pts with 1 vs ≥2 prior lines of therapy. Ixa dose was reduced in 57/307 pts (19%; n=2 not recorded); 246/309 pts (80%) discontinued Ixa, most frequently due to: progression in 37% of pts, adverse events (AEs) in 19%, and loss/lack of response in 10%. Among 215 pts who received ≥4 cycles of Ixa, rates of any‑grade (G) and G≥3 AEs were 62% and 37%; most common any-G AEs were thrombocytopenia (15%) and diarrhea (13%); the most common G≥3 AE was thrombocytopenia (6%).

Conclusion
UVEA-IXA data show that Ixa therapy is an effective, tolerable treatment option outside the clinical trial setting. Outcomes were favorable in pts with 1 vs ≥2 prior lines of therapy and ECOG PS 0–1 vs 2. Although UVEA-IXA data derive from retrospective chart review/infrequent prospective data capture and thus cannot be directly compared to clinical trial data, compared with TOURMALINE‑MM1 pts (Ixa arm; ORR 78%, mPFS 20.6 mos), UVEA-IXA pts had higher rates of ECOG PS 2 (20 vs 5%), ISS stage III MM (30 vs 12%), and eGFR <30 mL/min (5 vs 1%), and had received more prior lines of therapy (61 vs 38% had ≥2). Most common AEs were gastrointestinal and hematologic, consistent with the safety profile in TOURMALINE-MM1.
Keyword(s): Multiple myeloma, Proteasome inhibitor


