
Contributions
Abstract: EP990
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Ciltacabtagene autoleucel (cilta-cel; JNJ-68284528) is a chimeric antigen receptor T (CAR-T) cell therapy with two B-cell maturation antigen-targeting single-domain antibodies designed to confer avidity. CARTITUDE-1 (NCT03548207) is a single arm, phase 1b/2 study conducted to evaluate cilta-cel therapy in US patients with relapsed/refractory multiple myeloma (RRMM). Patients in CARTITUDE-1 were refractory to both an immunomodulatory imide drug (IMiD) and proteasome inhibitor (PI) or had received ≥3 prior lines of therapy and previous exposure to anti-CD38 monoclonal antibody (MoAb). While recent reports of cilta-cel efficacy were encouraging, it is unknown how they compare with similar patients receiving conventional (non CAR-T) treatment.
Aims
To compare the efficacy of cilta-cel versus conventional treatment in patients with RRMM.
Methods
A contemporary US-based dataset of patients with MM who were refractory to anti-CD38 MoAb (MAMMOTH study) was utilized to identify patients who would meet eligibility for CARTITUDE-1 and who received conventional therapy. Analyses were performed on the intent-to-treat (ITT) population in CARTITUDE-1, defined as patients who underwent apheresis (N=113), and a modified ITT (mITT) population, defined as the subset of patients who received cilta-cel at the recommended phase 2 dose (N=97). From the MAMMOTH dataset, we identified a population corresponding to CARTITUDE-1 ITT (N=190) and a mITT population, patients without death or progression within 47 days (median time between apheresis and cilta-cel infusion) from onset of therapy (N=122). Propensity scores (PS) were calculated with demographics, number of prior therapies, cytogenetics, and refractoriness to MM agents as covariates. Nearest neighbor 1:1 PS matching was performed by an analyst who was blinded to outcomes. The overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) were evaluated for ITT and mITT populations in CARTITUDE-1 vs. matching MAMMOTH cohorts.
Results
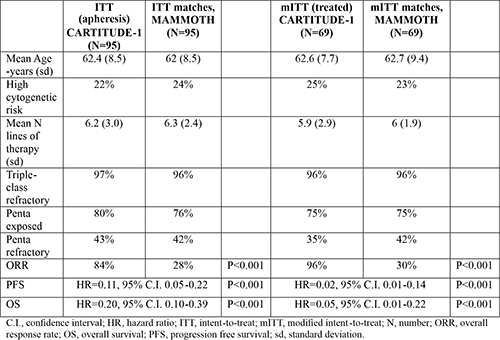
A total of 95 ITT (75 received bridging therapy, 82 received cilta-cel) and 69 mITT (54 received bridging) CARTITUDE-1 patients matched MAMMOTH patients (Table). Among the patients in the MAMMOTH ITT cohort, 34% had received pomalidomide, 24% anti-CD38 MoAb, 19% carfilzomib, and 35% cytotoxic chemotherapy in next therapy. ORR in the ITT cohorts was higher in CARTITUDE-1 than in MAMMOTH (84% vs. 28%). Compared with their MAMMOTH counterparts, patients in CARTITUDE-1 ITT cohort had improved PFS (12 months, 73% vs. 12%) and OS (12 months, 83% vs. 39%). Between the mITT cohorts, CARTITUDE-1 patients had superior ORR (96% vs. 30%), PFS (12 months, 79% vs. 15%), and OS (12 months, 88% vs. 41%).
Conclusion
In patients with RRMM beyond therapy with IMiD, PI, and anti-CD38 MoAb, treatment with cilta-cel is associated with higher response rate and superior PFS and OS when compared with conventional treatment.
Keyword(s): Antibody, B-cell maturation antigen, Multiple myeloma
Abstract: EP990
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Ciltacabtagene autoleucel (cilta-cel; JNJ-68284528) is a chimeric antigen receptor T (CAR-T) cell therapy with two B-cell maturation antigen-targeting single-domain antibodies designed to confer avidity. CARTITUDE-1 (NCT03548207) is a single arm, phase 1b/2 study conducted to evaluate cilta-cel therapy in US patients with relapsed/refractory multiple myeloma (RRMM). Patients in CARTITUDE-1 were refractory to both an immunomodulatory imide drug (IMiD) and proteasome inhibitor (PI) or had received ≥3 prior lines of therapy and previous exposure to anti-CD38 monoclonal antibody (MoAb). While recent reports of cilta-cel efficacy were encouraging, it is unknown how they compare with similar patients receiving conventional (non CAR-T) treatment.
Aims
To compare the efficacy of cilta-cel versus conventional treatment in patients with RRMM.
Methods
A contemporary US-based dataset of patients with MM who were refractory to anti-CD38 MoAb (MAMMOTH study) was utilized to identify patients who would meet eligibility for CARTITUDE-1 and who received conventional therapy. Analyses were performed on the intent-to-treat (ITT) population in CARTITUDE-1, defined as patients who underwent apheresis (N=113), and a modified ITT (mITT) population, defined as the subset of patients who received cilta-cel at the recommended phase 2 dose (N=97). From the MAMMOTH dataset, we identified a population corresponding to CARTITUDE-1 ITT (N=190) and a mITT population, patients without death or progression within 47 days (median time between apheresis and cilta-cel infusion) from onset of therapy (N=122). Propensity scores (PS) were calculated with demographics, number of prior therapies, cytogenetics, and refractoriness to MM agents as covariates. Nearest neighbor 1:1 PS matching was performed by an analyst who was blinded to outcomes. The overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) were evaluated for ITT and mITT populations in CARTITUDE-1 vs. matching MAMMOTH cohorts.
Results
A total of 95 ITT (75 received bridging therapy, 82 received cilta-cel) and 69 mITT (54 received bridging) CARTITUDE-1 patients matched MAMMOTH patients (Table). Among the patients in the MAMMOTH ITT cohort, 34% had received pomalidomide, 24% anti-CD38 MoAb, 19% carfilzomib, and 35% cytotoxic chemotherapy in next therapy. ORR in the ITT cohorts was higher in CARTITUDE-1 than in MAMMOTH (84% vs. 28%). Compared with their MAMMOTH counterparts, patients in CARTITUDE-1 ITT cohort had improved PFS (12 months, 73% vs. 12%) and OS (12 months, 83% vs. 39%). Between the mITT cohorts, CARTITUDE-1 patients had superior ORR (96% vs. 30%), PFS (12 months, 79% vs. 15%), and OS (12 months, 88% vs. 41%).

Conclusion
In patients with RRMM beyond therapy with IMiD, PI, and anti-CD38 MoAb, treatment with cilta-cel is associated with higher response rate and superior PFS and OS when compared with conventional treatment.
Keyword(s): Antibody, B-cell maturation antigen, Multiple myeloma


