
Contributions
Abstract: EP988
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Patients (pts) with newly diagnosed multiple myeloma (MM) are routinely treated with lenalidomide (LEN) until disease progression. Thus, at the time of first relapse, many pts will have LEN-refractory disease. These pts need proven effective therapies that not only help achieve disease control but also have a manageable safety profile. Pts with relapsed or refractory MM (RRMM) who were treated with pomalidomide (POM), bortezomib (BORT), and dexamethasone (DEX; PVd) at first relapse in OPTIMISMM (phase 3, NT01734928) had a significantly improved median PFS (20.7 vs 11.6 mo; HR, 0.54; 95% CI, 0.36-0.82; P = .0027) vs Vd; all pts were previously treated with LEN, and 57% were LEN refractory (Richardson PG, et al. Lancet Oncol 2019;20:781-794). Adverse events reported with PVd in the overall population were consistent with the individual safety profiles for POM, BORT, and DEX.
Aims
To report a safety analysis of treatment-emergent adverse events (TEAEs) in the events of interest (EOI) category for PVd vs Vd administered at first relapse in OPTIMISMM.
Methods
In 21-day cycles, pts received PVd or Vd (1:1). POM 4 mg/d was given on d 1-14 (PVd arm only); BORT 1.3 mg/m2 was given on d 1, 4, 8, and 11 of cycle 1-8 and d 1 and 8 of cycle 9+; and DEX 20 mg/d (10 mg/d for pts aged > 75 yr) was given on the days of and after BORT. Key eligibility criteria included ≥ 2 cycles of prior LEN; LEN-refractory pts were allowed. Adverse events were graded according to the NCI CTCAE (version 4.0) and were summarized by system organ class and preferred term. All pts provided informed consent.
Results
226 of 559 pts (40%) in OPTIMISMM had 1 prior line of therapy (LOT) and 221 (PVd, n=111; Vd, n=110) were included in the safety analysis population (all pts who received ≥1 dose of study drug). Baseline characteristics were generally well balanced between Tx arms. All pts had prior LEN Tx and 57% (126/221) were LEN refractory; 59% (131/221) had prior BORT Tx. Median duration of treatment was longer in the PVd vs Vd arm: 47.4 wk (4.7-147.0) vs 27.1 wk (0.4-162.0). Grade (Gr) 3/4 TEAEs EOI occurred in 57.5% of pts (PVd, 69.4%; Vd, 45.5%; Table). Overall, the most common Gr 3/4 TEAEs EOI were neutropenia (24.4%), infections and infestations (22.2%), and thrombocytopenia (20.4%). Gr 3/4 TEAEs EOI more common in the PVd vs Vd arm included neutropenia (36.9% vs 11.8%), infections and infestations (28.8% vs 15.5%), and peripheral neuropathy (11.7% vs 4.5%). Onset of neutropenia was mostly in the first 3 cycles and primarily Gr 3/4. Onset of infections and infestations commonly occurred during cycles 1-9 and were mostly Gr 1/2. Onset of peripheral neuropathy commonly occurred in the first 6 cycles; instances were mostly Gr 1/2. See table for median time to onset and duration of selected TEAE EOIs. TEAE EOIs were mainly managed with dose reductions and interruptions (Table). POM discontinuations due to ≥1 TEAE EOI of any grade were low (9.0%). In the PVd arm, 62% (31/50) of pts with any-grade neutropenia received G-CSF support. No pt discontinued POM due to neutropenia. Infections and infestations were the primary cause of PVd dose interruptions (54.1%). Peripheral neuropathy was the most common TEAE EOI leading to dose reduction (32.4%) and drug discontinuation (16.2%) with PVd.
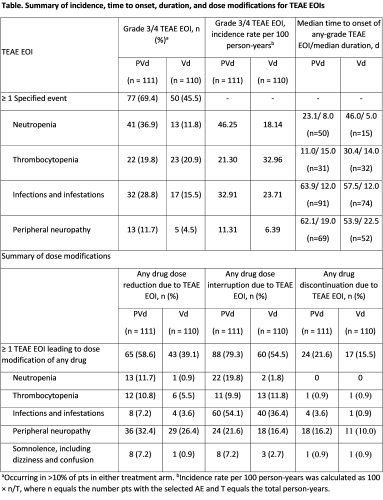
Conclusion
The safety profile for PVd at first relapse in pts with MM is consistent with that in previous reports. TEAEs EOI generally occurred in early cycles of treatment and could be managed with dose modifications or appropriate treatment for infections.
Keyword(s): Multiple myeloma, Refractory, Relapse, Safety
Abstract: EP988
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Patients (pts) with newly diagnosed multiple myeloma (MM) are routinely treated with lenalidomide (LEN) until disease progression. Thus, at the time of first relapse, many pts will have LEN-refractory disease. These pts need proven effective therapies that not only help achieve disease control but also have a manageable safety profile. Pts with relapsed or refractory MM (RRMM) who were treated with pomalidomide (POM), bortezomib (BORT), and dexamethasone (DEX; PVd) at first relapse in OPTIMISMM (phase 3, NT01734928) had a significantly improved median PFS (20.7 vs 11.6 mo; HR, 0.54; 95% CI, 0.36-0.82; P = .0027) vs Vd; all pts were previously treated with LEN, and 57% were LEN refractory (Richardson PG, et al. Lancet Oncol 2019;20:781-794). Adverse events reported with PVd in the overall population were consistent with the individual safety profiles for POM, BORT, and DEX.
Aims
To report a safety analysis of treatment-emergent adverse events (TEAEs) in the events of interest (EOI) category for PVd vs Vd administered at first relapse in OPTIMISMM.
Methods
In 21-day cycles, pts received PVd or Vd (1:1). POM 4 mg/d was given on d 1-14 (PVd arm only); BORT 1.3 mg/m2 was given on d 1, 4, 8, and 11 of cycle 1-8 and d 1 and 8 of cycle 9+; and DEX 20 mg/d (10 mg/d for pts aged > 75 yr) was given on the days of and after BORT. Key eligibility criteria included ≥ 2 cycles of prior LEN; LEN-refractory pts were allowed. Adverse events were graded according to the NCI CTCAE (version 4.0) and were summarized by system organ class and preferred term. All pts provided informed consent.
Results
226 of 559 pts (40%) in OPTIMISMM had 1 prior line of therapy (LOT) and 221 (PVd, n=111; Vd, n=110) were included in the safety analysis population (all pts who received ≥1 dose of study drug). Baseline characteristics were generally well balanced between Tx arms. All pts had prior LEN Tx and 57% (126/221) were LEN refractory; 59% (131/221) had prior BORT Tx. Median duration of treatment was longer in the PVd vs Vd arm: 47.4 wk (4.7-147.0) vs 27.1 wk (0.4-162.0). Grade (Gr) 3/4 TEAEs EOI occurred in 57.5% of pts (PVd, 69.4%; Vd, 45.5%; Table). Overall, the most common Gr 3/4 TEAEs EOI were neutropenia (24.4%), infections and infestations (22.2%), and thrombocytopenia (20.4%). Gr 3/4 TEAEs EOI more common in the PVd vs Vd arm included neutropenia (36.9% vs 11.8%), infections and infestations (28.8% vs 15.5%), and peripheral neuropathy (11.7% vs 4.5%). Onset of neutropenia was mostly in the first 3 cycles and primarily Gr 3/4. Onset of infections and infestations commonly occurred during cycles 1-9 and were mostly Gr 1/2. Onset of peripheral neuropathy commonly occurred in the first 6 cycles; instances were mostly Gr 1/2. See table for median time to onset and duration of selected TEAE EOIs. TEAE EOIs were mainly managed with dose reductions and interruptions (Table). POM discontinuations due to ≥1 TEAE EOI of any grade were low (9.0%). In the PVd arm, 62% (31/50) of pts with any-grade neutropenia received G-CSF support. No pt discontinued POM due to neutropenia. Infections and infestations were the primary cause of PVd dose interruptions (54.1%). Peripheral neuropathy was the most common TEAE EOI leading to dose reduction (32.4%) and drug discontinuation (16.2%) with PVd.
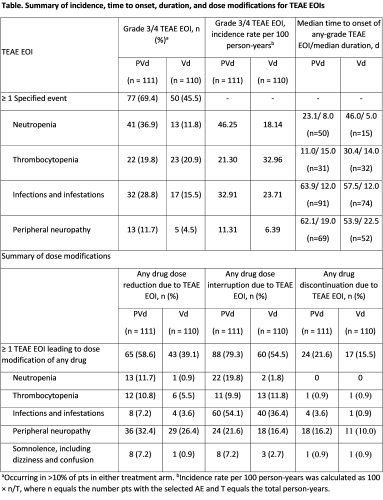
Conclusion
The safety profile for PVd at first relapse in pts with MM is consistent with that in previous reports. TEAEs EOI generally occurred in early cycles of treatment and could be managed with dose modifications or appropriate treatment for infections.
Keyword(s): Multiple myeloma, Refractory, Relapse, Safety


