
Contributions
Abstract: EP984
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell therapy, showed promising efficacy in patients with relapsed and refractory multiple myeloma (RRMM) in the KarMMa study with an overall response rate (ORR) of 73% and a complete response rate (CRR) of 33% across the target dose range of 150–450 × 106 CAR+ T cells (Munshi NC, et al. N Engl J Med 2021;384:705-716). CAR T cell therapies have been associated with potentially severe adverse events, including cytokine release syndrome (CRS) and neurotoxicity (NT). Improved understanding of these adverse events may allow for better management of such toxicities.
Aims
To examine associations of NT with patient and disease characteristics, describe patient management, and evaluate the impact of NT on outcomes in the KarMMa study.
Methods
Patients enrolled in the KarMMa study had received ≥3 prior regimens, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody, and were refractory to their last regimen per IMWG criteria (NCT03361748). Ide-cel target doses of 150, 300, or 450 × 106 CAR+ T cells were given after 3 d of lymphodepletion with cyclophosphamide (300 mg/m2/d) and fludarabine (30 mg/m2/d) followed by 2 d of rest. Endpoints included ORR (primary), CRR (key secondary), duration of response (DOR), progression-free survival, and safety. Investigator-identified NT events were captured under the single preferred term of neurotoxicity and graded according to NCI CTCAE v4.03. Events were managed with corticosteroids, anakinra, and tocilizumab as needed.
Results
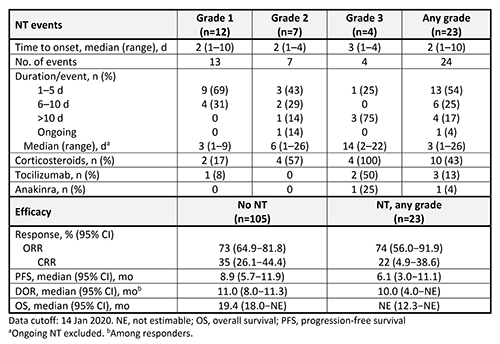
Of 128 patients treated with ide-cel, NT was reported in 23 (18%); 12 patients (9%) had maximum grade 1 NT, 7 (5%) had grade 2, and 4 (3%) had grade 3. No grade 4 or 5 NT occurred. Most baseline characteristics were similar in patients with and without NT, including R-ISS disease stage III (22% and 15%), high-risk cytogenetics (39% and 34%), and extramedullary disease (35% and 40%); exceptions were high tumor burden (65% and 48%) and sex (48% and 62% men). NT (any grade/grade 3) occurred in 0%/0%, 17%/1%, and 20%/6% of patients at target doses of 150, 300, and 450 × 106 CAR+ T cells. Median time to onset was similar regardless of grade, and there were no late-onset events (Table). NT was managed with corticosteroids in 10 patients (43%), tocilizumab in 3 (13%), and anakinra in 1 (4%). Median (range) time to first use of corticosteroids was 2 d (1–6). ORR and DOR were similar in patients with and without NT (Table). All NT occurred in the proximity of CRS events with the start date of NT events either overlapping with or occurring within 1 wk of the start of a CRS event.
Conclusion
NT occurred early in the KarMMa study, was generally of short duration, and was mostly grade 1/2 with no grade ≥4 events. All NT was proximal to CRS. Patients with NT had a favorable ORR after ide-cel treatment. These results continue to demonstrate the durable efficacy and tolerability of ide-cel in patients with RRMM.
Keyword(s): CAR-T, Multiple myeloma, Toxicity
Abstract: EP984
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell therapy, showed promising efficacy in patients with relapsed and refractory multiple myeloma (RRMM) in the KarMMa study with an overall response rate (ORR) of 73% and a complete response rate (CRR) of 33% across the target dose range of 150–450 × 106 CAR+ T cells (Munshi NC, et al. N Engl J Med 2021;384:705-716). CAR T cell therapies have been associated with potentially severe adverse events, including cytokine release syndrome (CRS) and neurotoxicity (NT). Improved understanding of these adverse events may allow for better management of such toxicities.
Aims
To examine associations of NT with patient and disease characteristics, describe patient management, and evaluate the impact of NT on outcomes in the KarMMa study.
Methods
Patients enrolled in the KarMMa study had received ≥3 prior regimens, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody, and were refractory to their last regimen per IMWG criteria (NCT03361748). Ide-cel target doses of 150, 300, or 450 × 106 CAR+ T cells were given after 3 d of lymphodepletion with cyclophosphamide (300 mg/m2/d) and fludarabine (30 mg/m2/d) followed by 2 d of rest. Endpoints included ORR (primary), CRR (key secondary), duration of response (DOR), progression-free survival, and safety. Investigator-identified NT events were captured under the single preferred term of neurotoxicity and graded according to NCI CTCAE v4.03. Events were managed with corticosteroids, anakinra, and tocilizumab as needed.
Results
Of 128 patients treated with ide-cel, NT was reported in 23 (18%); 12 patients (9%) had maximum grade 1 NT, 7 (5%) had grade 2, and 4 (3%) had grade 3. No grade 4 or 5 NT occurred. Most baseline characteristics were similar in patients with and without NT, including R-ISS disease stage III (22% and 15%), high-risk cytogenetics (39% and 34%), and extramedullary disease (35% and 40%); exceptions were high tumor burden (65% and 48%) and sex (48% and 62% men). NT (any grade/grade 3) occurred in 0%/0%, 17%/1%, and 20%/6% of patients at target doses of 150, 300, and 450 × 106 CAR+ T cells. Median time to onset was similar regardless of grade, and there were no late-onset events (Table). NT was managed with corticosteroids in 10 patients (43%), tocilizumab in 3 (13%), and anakinra in 1 (4%). Median (range) time to first use of corticosteroids was 2 d (1–6). ORR and DOR were similar in patients with and without NT (Table). All NT occurred in the proximity of CRS events with the start date of NT events either overlapping with or occurring within 1 wk of the start of a CRS event.

Conclusion
NT occurred early in the KarMMa study, was generally of short duration, and was mostly grade 1/2 with no grade ≥4 events. All NT was proximal to CRS. Patients with NT had a favorable ORR after ide-cel treatment. These results continue to demonstrate the durable efficacy and tolerability of ide-cel in patients with RRMM.
Keyword(s): CAR-T, Multiple myeloma, Toxicity


