
Contributions
Abstract: EP983
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Plasma cell disorder (PCD) patients are extremely vulnerable to SARS-CoV-2 infection due to disease-related impaired humoral and cellular immunity as well as the receipt of immunosuppressive therapy. Reported mortality in a large cohort of patients with plasma cell disorders is 33%. The roll out of the COVID-19 vaccine is welcomed in this population, however there is concern of suboptimal antibody responses, from previous experience with the influenza vaccine. There is urgent need to understand the humoral response to SARS-CoV-2 infection in these patients, in the context of systemic anti-cancer therapy (SACT).
Aims
We aimed to investigate the presence of SARS-CoV-2 antibodies in a cohort of PCD patients, the relationship with symptomatic infection, PCD characteristics and receipt of SACT.
Methods
SARS-CoV-2 antibody screening with the Elecsys Anti-SARS-CoV-2 assay (Roche Diagnostics, Basel, Switzerland), a semi-quantitative assay of IgG and IgM against the nucleocapsid (N) antigen was introduced for PCD patients at our institution in July 2020. Clinical information was retrieved from the medical records. Patients with unexpected positive antibody tests were asked about possible past contacts and exposure to SARS-CoV-2.
Results
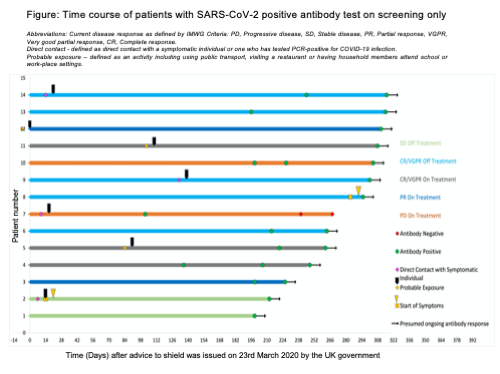
We report on a six-month period of routine SARS-CoV-2 antibody screening. Two-hundred and forty-three PCD patients had one antibody test, 106 had serial samples. Total seroprevalence was 10.7% (26/243), of which 12 were patients with known PCR-swab positive COVID-19 disease. In a separate but overlapping cohort, 41 patients have had PCR confirmed COVID-19 disease; 20 of these patients were tested, and 12 (60%) had seroconverted. Median time to testing from positive PCR test in the antibody positive patients was 86.5 days (range 22-256) and in antibody negative patients, 30.5 days (range 5-176 days). No PCD or COVID-19 disease factors were found to influence the likelihood of mounting an antibody response after PCR-confirmed COVID-19 disease in these 20 patients. In our screened cohort, 14 (6.3%) patients were unexpectedly antibody positive. Their clinical course is summarised in the included figure. The majority 85.7% (12/14) of patients described no COVID-19 symptoms. Seven (50%) patients were on SACT (including ixazomib, pomalidomide, lenalidomide and dexamethasone combinations) throughout the period from possible exposure to positive antibody test, with no interruption to their ongoing oral immunomodulatory treatment. Ten antibody positive patients had serial positive results at median 45 days (range 21-119) apart, demonstrating persistence, but some decline in titre over time.
Conclusion
Our seroprevalence of 10.7% is lower but not dissimilar to that reported in the London population over a similar time period reflecting shielding behaviours in our patients but also the challenges of protecting them during high SARS-CoV-2 incidence in the community. Nevertheless, PCD patients retain the ability to seroconvert, even with asymptomatic COVID-19 disease and while on immunomodulatory therapy. Seroconversion rates following symptomatic infection appear lower however, with evidence of delay compared to the general population. These data support the advice for COVID-19 vaccination to be offered to all PCD patients although the suboptimal humoral response calls for close antibody monitoring of all vaccinated PCD patients and timely booster doses.
Keyword(s): Amyloidosis, Antibody response, COVID-19, Multiple myeloma
Abstract: EP983
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Plasma cell disorder (PCD) patients are extremely vulnerable to SARS-CoV-2 infection due to disease-related impaired humoral and cellular immunity as well as the receipt of immunosuppressive therapy. Reported mortality in a large cohort of patients with plasma cell disorders is 33%. The roll out of the COVID-19 vaccine is welcomed in this population, however there is concern of suboptimal antibody responses, from previous experience with the influenza vaccine. There is urgent need to understand the humoral response to SARS-CoV-2 infection in these patients, in the context of systemic anti-cancer therapy (SACT).
Aims
We aimed to investigate the presence of SARS-CoV-2 antibodies in a cohort of PCD patients, the relationship with symptomatic infection, PCD characteristics and receipt of SACT.
Methods
SARS-CoV-2 antibody screening with the Elecsys Anti-SARS-CoV-2 assay (Roche Diagnostics, Basel, Switzerland), a semi-quantitative assay of IgG and IgM against the nucleocapsid (N) antigen was introduced for PCD patients at our institution in July 2020. Clinical information was retrieved from the medical records. Patients with unexpected positive antibody tests were asked about possible past contacts and exposure to SARS-CoV-2.
Results
We report on a six-month period of routine SARS-CoV-2 antibody screening. Two-hundred and forty-three PCD patients had one antibody test, 106 had serial samples. Total seroprevalence was 10.7% (26/243), of which 12 were patients with known PCR-swab positive COVID-19 disease. In a separate but overlapping cohort, 41 patients have had PCR confirmed COVID-19 disease; 20 of these patients were tested, and 12 (60%) had seroconverted. Median time to testing from positive PCR test in the antibody positive patients was 86.5 days (range 22-256) and in antibody negative patients, 30.5 days (range 5-176 days). No PCD or COVID-19 disease factors were found to influence the likelihood of mounting an antibody response after PCR-confirmed COVID-19 disease in these 20 patients. In our screened cohort, 14 (6.3%) patients were unexpectedly antibody positive. Their clinical course is summarised in the included figure. The majority 85.7% (12/14) of patients described no COVID-19 symptoms. Seven (50%) patients were on SACT (including ixazomib, pomalidomide, lenalidomide and dexamethasone combinations) throughout the period from possible exposure to positive antibody test, with no interruption to their ongoing oral immunomodulatory treatment. Ten antibody positive patients had serial positive results at median 45 days (range 21-119) apart, demonstrating persistence, but some decline in titre over time.

Conclusion
Our seroprevalence of 10.7% is lower but not dissimilar to that reported in the London population over a similar time period reflecting shielding behaviours in our patients but also the challenges of protecting them during high SARS-CoV-2 incidence in the community. Nevertheless, PCD patients retain the ability to seroconvert, even with asymptomatic COVID-19 disease and while on immunomodulatory therapy. Seroconversion rates following symptomatic infection appear lower however, with evidence of delay compared to the general population. These data support the advice for COVID-19 vaccination to be offered to all PCD patients although the suboptimal humoral response calls for close antibody monitoring of all vaccinated PCD patients and timely booster doses.
Keyword(s): Amyloidosis, Antibody response, COVID-19, Multiple myeloma


