Contributions
Abstract: EP982
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Lenalidomide (Len)- and bortezomib-based treatments are standard frontline therapies for multiple myeloma (MM). Many patients discontinue these drugs due to progression or toxicity, resulting in a need for bortezomib- and len-sparing treatment combinations for relapsed or refractory (RR) patients. After approximately 27 months of median follow-up in the phase 3, randomized CANDOR study, median progression-free survival was 29 months in patients treated with carfilzomib, dexamethasone, and daratumumab (KdD) vs 15 months in patients treated with carfilzomib and dexamethasone (Kd) (hazard ratio [HR]: 0.59; 95% confidence interval [CI]: 0.45–0.78) (Dimopoulos et al., Blood 2020).
Aims
To compare time to next treatment (TTNT), time to progression (TTP), and time to second objective disease progression (PFS2) in patients treated with KdD vs Kd in the CANDOR study.
Methods
The study design of CANDOR (NCT03158688) has been previously published (Dimopoulos et al., Lancet 2020). This post hoc analysis used a planned interim readout from CANDOR (data cutoff: June 15, 2020). TTNT was defined as the time from randomization to initiation of subsequent MM treatments. Patients who did not move on to a next treatment were censored. TTP was defined as the time from randomization to disease progression as assessed by a validated computer algorithm (Onyx Response Computer Algorithm). PFS2 was defined as the time from randomization to second disease progression or death, whichever occurred first. Since the date of the second disease progression was not collected in CANDOR, for patients who had progressive disease (PD), the start date of the next line of therapy was used as a surrogate for the PFS2 event; for patients who had not had a PD, the start date of the third line of therapy was used as a surrogate for the PFS2 event. TTNT, TTP, and PFS2 were compared between KdD and Kd arms using a stratified log-rank test and HRs were estimated by a stratified Cox proportional hazards model with stratification factors of International Staging System stage (1 or 2 vs 3), prior proteasome inhibitor (PI) exposure, and number of prior lines of therapy.
Results
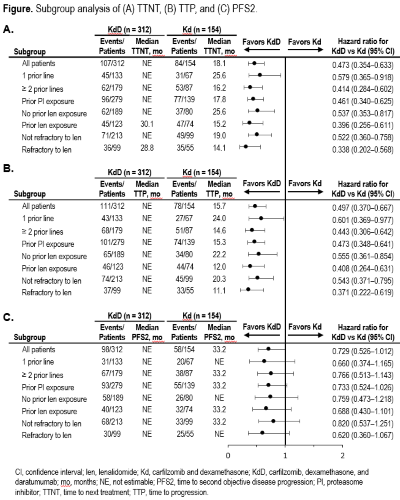
Patients treated with KdD had longer TTNT compared with patients treated with Kd (HR: 0.473; 95% CI: 0.354–0.633), with median TTNT for patients in the KdD arm not estimable (NE) (95% CI: 30–NE) vs 18 months (95% CI: 14–25) in the Kd arm. Treatment with KdD improved TTP compared with Kd treatment (HR: 0.497; 95% CI: 0.370–0.667); median TTP was NE (95% CI: 28–NE) in the KdD arm vs 16 months (95% CI: 12–22) in the Kd arm. Median PFS2 was NE (95% CI: NE–NE) in the KdD arm vs 33 months (95% CI: 33–NE) in the Kd arm (HR: 0.729; 95% CI: 0.526–1.012). The observed improvements in TTNT, TTP, and PFS2 for KdD vs Kd-treated patients were consistent regardless of prior lines of therapy, prior PI exposure, prior len exposure, and len-refractoriness (Figure).
Conclusion
Patients receiving KdD vs Kd had improvements in time to their next treatment, time to disease progression, and time to second disease progression or death following subsequent therapies. These data confirm and expand upon previously reported results of CANDOR, indicating the robust efficacy benefit of KdD across clinically relevant subgroups, including in those exposed and/or refractory to len. These findings reinforce the clinical value of KdD as an emerging standard of care in the management of RRMM for patients with 1–3 prior lines of therapy.
Keyword(s): Multiple myeloma, Proteasome inhibitor
Abstract: EP982
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Lenalidomide (Len)- and bortezomib-based treatments are standard frontline therapies for multiple myeloma (MM). Many patients discontinue these drugs due to progression or toxicity, resulting in a need for bortezomib- and len-sparing treatment combinations for relapsed or refractory (RR) patients. After approximately 27 months of median follow-up in the phase 3, randomized CANDOR study, median progression-free survival was 29 months in patients treated with carfilzomib, dexamethasone, and daratumumab (KdD) vs 15 months in patients treated with carfilzomib and dexamethasone (Kd) (hazard ratio [HR]: 0.59; 95% confidence interval [CI]: 0.45–0.78) (Dimopoulos et al., Blood 2020).
Aims
To compare time to next treatment (TTNT), time to progression (TTP), and time to second objective disease progression (PFS2) in patients treated with KdD vs Kd in the CANDOR study.
Methods
The study design of CANDOR (NCT03158688) has been previously published (Dimopoulos et al., Lancet 2020). This post hoc analysis used a planned interim readout from CANDOR (data cutoff: June 15, 2020). TTNT was defined as the time from randomization to initiation of subsequent MM treatments. Patients who did not move on to a next treatment were censored. TTP was defined as the time from randomization to disease progression as assessed by a validated computer algorithm (Onyx Response Computer Algorithm). PFS2 was defined as the time from randomization to second disease progression or death, whichever occurred first. Since the date of the second disease progression was not collected in CANDOR, for patients who had progressive disease (PD), the start date of the next line of therapy was used as a surrogate for the PFS2 event; for patients who had not had a PD, the start date of the third line of therapy was used as a surrogate for the PFS2 event. TTNT, TTP, and PFS2 were compared between KdD and Kd arms using a stratified log-rank test and HRs were estimated by a stratified Cox proportional hazards model with stratification factors of International Staging System stage (1 or 2 vs 3), prior proteasome inhibitor (PI) exposure, and number of prior lines of therapy.
Results
Patients treated with KdD had longer TTNT compared with patients treated with Kd (HR: 0.473; 95% CI: 0.354–0.633), with median TTNT for patients in the KdD arm not estimable (NE) (95% CI: 30–NE) vs 18 months (95% CI: 14–25) in the Kd arm. Treatment with KdD improved TTP compared with Kd treatment (HR: 0.497; 95% CI: 0.370–0.667); median TTP was NE (95% CI: 28–NE) in the KdD arm vs 16 months (95% CI: 12–22) in the Kd arm. Median PFS2 was NE (95% CI: NE–NE) in the KdD arm vs 33 months (95% CI: 33–NE) in the Kd arm (HR: 0.729; 95% CI: 0.526–1.012). The observed improvements in TTNT, TTP, and PFS2 for KdD vs Kd-treated patients were consistent regardless of prior lines of therapy, prior PI exposure, prior len exposure, and len-refractoriness (Figure).
Conclusion
Patients receiving KdD vs Kd had improvements in time to their next treatment, time to disease progression, and time to second disease progression or death following subsequent therapies. These data confirm and expand upon previously reported results of CANDOR, indicating the robust efficacy benefit of KdD across clinically relevant subgroups, including in those exposed and/or refractory to len. These findings reinforce the clinical value of KdD as an emerging standard of care in the management of RRMM for patients with 1–3 prior lines of therapy.
Keyword(s): Multiple myeloma, Proteasome inhibitor