
Contributions
Abstract: EP981
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
A prespecified interim efficacy analysis of the Phase 3 IKEMA study (NCT03275285) demonstrated that isatuximab (Isa) plus carfilzomib (K) and dexamethasone (d) (Isa-Kd) significantly improved progression-free survival (PFS) compared with Kd in patients (pts) with relapsed multiple myeloma (RMM) (HR 0.531; 99% CI, 0.318–0.889; P=0.0007), with a clinically meaningful increase in minimal residual disease negativity (MRD-) (29.6% vs 13.0%) and complete response (CR) (39.7% vs 27.6%) rates, and a manageable safety profile.
Aims
This subgroup analysis of IKEMA examined efficacy and safety in pts with high-risk cytogenetics [t(4;14), del(17p), and t(14;16)] and/or gain(1q21).
Methods
Pts with 1–3 prior lines of therapy were randomized 3:2 to receive Isa-Kd (n=179) or Kd (n=123). High-risk cytogenetics was assessed by central laboratory analysis and defined as ≥1 of the following: del(17p): 50% cutoff; t(4;14) or t(14;16): 30% cutoff. Assessment of gain(1q21) was prespecified as ≥3 copies: 30% cutoff.
Results
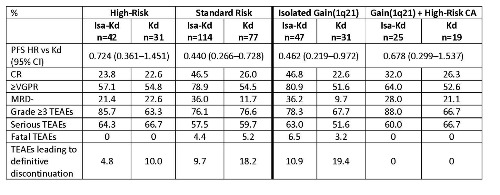
Of the randomized pts, 23.5% (Isa-Kd) and 25.2% (Kd) had ≥1 high-risk cytogenetic abnormality (CA); 26.3% (Isa-Kd) and 25.2% (Kd) had isolated gain(1q21). The addition of Isa to Kd improved PFS for pts with ≥1 high-risk CA and standard-risk pts (Table); pts with t(4;14) (HR 0.549; 95% CI, 0.232–1.301) had a more pronounced treatment effect than pts with del(17p) (HR 0.837; 95% CI, 0.281–2.496). A clear PFS benefit with Isa-Kd was also seen for pts with isolated gain(1q21) and gain(1q21) combined with other high-risk CA (Table). The trend toward improved CR, ≥very good partial response (VGPR), and MRD- rates with the addition of Isa was more pronounced in pts with gain(1q21) than in pts with high-risk CA alone. Grade ≥3 treatment-emergent adverse events (TEAEs) were more common with Isa-Kd vs Kd, but the incidence of serious and fatal TEAEs was similar with both arms for high-risk pts (Table).
Conclusion
The addition of Isa to Kd improved PFS in pts with high-risk CA and disease response in pts with gain(1q21) isolated or combined with high-risk CA, with a manageable safety profile, consistent with the benefit observed in the overall IKEMA population. Isa-Kd is a potential new treatment option for the difficult-to-treat subgroup of pts with RMM and high-risk cytogenetics.
Keyword(s): CD38, Monoclonal antibody, Multiple myeloma, Phase III
Abstract: EP981
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
A prespecified interim efficacy analysis of the Phase 3 IKEMA study (NCT03275285) demonstrated that isatuximab (Isa) plus carfilzomib (K) and dexamethasone (d) (Isa-Kd) significantly improved progression-free survival (PFS) compared with Kd in patients (pts) with relapsed multiple myeloma (RMM) (HR 0.531; 99% CI, 0.318–0.889; P=0.0007), with a clinically meaningful increase in minimal residual disease negativity (MRD-) (29.6% vs 13.0%) and complete response (CR) (39.7% vs 27.6%) rates, and a manageable safety profile.
Aims
This subgroup analysis of IKEMA examined efficacy and safety in pts with high-risk cytogenetics [t(4;14), del(17p), and t(14;16)] and/or gain(1q21).
Methods
Pts with 1–3 prior lines of therapy were randomized 3:2 to receive Isa-Kd (n=179) or Kd (n=123). High-risk cytogenetics was assessed by central laboratory analysis and defined as ≥1 of the following: del(17p): 50% cutoff; t(4;14) or t(14;16): 30% cutoff. Assessment of gain(1q21) was prespecified as ≥3 copies: 30% cutoff.
Results
Of the randomized pts, 23.5% (Isa-Kd) and 25.2% (Kd) had ≥1 high-risk cytogenetic abnormality (CA); 26.3% (Isa-Kd) and 25.2% (Kd) had isolated gain(1q21). The addition of Isa to Kd improved PFS for pts with ≥1 high-risk CA and standard-risk pts (Table); pts with t(4;14) (HR 0.549; 95% CI, 0.232–1.301) had a more pronounced treatment effect than pts with del(17p) (HR 0.837; 95% CI, 0.281–2.496). A clear PFS benefit with Isa-Kd was also seen for pts with isolated gain(1q21) and gain(1q21) combined with other high-risk CA (Table). The trend toward improved CR, ≥very good partial response (VGPR), and MRD- rates with the addition of Isa was more pronounced in pts with gain(1q21) than in pts with high-risk CA alone. Grade ≥3 treatment-emergent adverse events (TEAEs) were more common with Isa-Kd vs Kd, but the incidence of serious and fatal TEAEs was similar with both arms for high-risk pts (Table).

Conclusion
The addition of Isa to Kd improved PFS in pts with high-risk CA and disease response in pts with gain(1q21) isolated or combined with high-risk CA, with a manageable safety profile, consistent with the benefit observed in the overall IKEMA population. Isa-Kd is a potential new treatment option for the difficult-to-treat subgroup of pts with RMM and high-risk cytogenetics.
Keyword(s): CD38, Monoclonal antibody, Multiple myeloma, Phase III


