
Contributions
Abstract: EP979
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Lenalidomide plus low-dose dexamethasone (Rd) is an established standard of care for transplant-ineligible patients (pts) with newly diagnosed multiple myeloma (NDMM) of all ages. Approval was based on the results of the pivotal phase III FIRST trial showing a significantly prolonged progression-free (PFS) and overall survival (OS) in pts treated with Rd compared with melphalan-prednisone-thalidomide (MPT) (Facon et al., Blood 2018). Since MM is a diagnosis of advanced age and only 35% of pts were age older than 75 years in the FIRST trial, the results are difficult to extrapolate to a real-world (RW) setting. Besides, RW data of this commonly used regimen are still scarce.
Aims
The focus of the final analysis part one of the FIRST-NIS is to evaluate effectiveness and safety of first-line Rd therapy in NDMM pts in a RW setting.
Methods
The prospective, multicenter, non-interventional, observational FIRST-NIS study collected data on 172 MM pts receiving Rd first-line therapy from 41 sites across Germany. Primary objective was to evaluate effectiveness of Rd assessed by 24-month PFS rate (PFS24). Secondary endpoints included OS, PFS, objective response rate (ORR), duration of response, quality of life (QoL) and safety. Descriptive statistics were used to analyze the data. Time-to-event analyses were performed using the Kaplan-Meier method. Final survival analysis will be performed 60 months after last patient in (LPI) in 2023 (final analysis part two).
Results
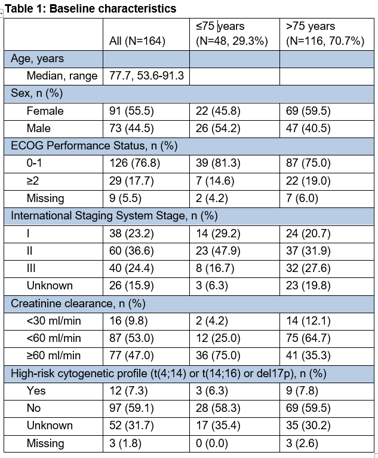
Of 172 pts enrolled, 164 pts qualified for effectiveness and 168 pts for safety analyses. Baseline characteristics of all pts and pts stratified by age (≤75, >75 years) are presented in Table 1. With a median follow-up of 18.7 months at data base cut (4th November 2020), PFS24 rate was 48.0% for the total cohort, 65.5% for pts ≤75 years and 40.1% for pts >75 years. The median PFS was 22.9 months for all pts, 31.5 months for pts ≤75 years and 19.3 months for pts >75 years. The ORR was 59.1% in all pts, 70.8% in pts ≤75 years and 54.3% in pts >75 years. In total, 98.2% of pts developed adverse events (AEs). Grade 3/4 AEs were detected in 63.1% of pts, of which the most common were anemia (8.3%), thrombocytopenia (4.8%), dyspnea (4.8%) and pneumonia (4.8%).
Conclusion
Although the FIRST-NIS represents an elderly RW pts population with approximately 70% of pts aged >75 years, the results revealed a favorable risk-benefit profile for Rd in NDMM pts. In elderly pts >75 years, however, PFS results were lower compared to younger pts ≤75 years. Reasons might be the higher rates of advanced-stage disease and renal impairment observed in the elderly pts. Overall, the Rd combination seems to be a reasonable and orally available treatment option also for elderly pts with NDMM in the RW setting.
Keyword(s): Dexamethasone, Immunomodulatory thalidomide analog, Multiple myeloma
Abstract: EP979
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Lenalidomide plus low-dose dexamethasone (Rd) is an established standard of care for transplant-ineligible patients (pts) with newly diagnosed multiple myeloma (NDMM) of all ages. Approval was based on the results of the pivotal phase III FIRST trial showing a significantly prolonged progression-free (PFS) and overall survival (OS) in pts treated with Rd compared with melphalan-prednisone-thalidomide (MPT) (Facon et al., Blood 2018). Since MM is a diagnosis of advanced age and only 35% of pts were age older than 75 years in the FIRST trial, the results are difficult to extrapolate to a real-world (RW) setting. Besides, RW data of this commonly used regimen are still scarce.
Aims
The focus of the final analysis part one of the FIRST-NIS is to evaluate effectiveness and safety of first-line Rd therapy in NDMM pts in a RW setting.
Methods
The prospective, multicenter, non-interventional, observational FIRST-NIS study collected data on 172 MM pts receiving Rd first-line therapy from 41 sites across Germany. Primary objective was to evaluate effectiveness of Rd assessed by 24-month PFS rate (PFS24). Secondary endpoints included OS, PFS, objective response rate (ORR), duration of response, quality of life (QoL) and safety. Descriptive statistics were used to analyze the data. Time-to-event analyses were performed using the Kaplan-Meier method. Final survival analysis will be performed 60 months after last patient in (LPI) in 2023 (final analysis part two).
Results
Of 172 pts enrolled, 164 pts qualified for effectiveness and 168 pts for safety analyses. Baseline characteristics of all pts and pts stratified by age (≤75, >75 years) are presented in Table 1. With a median follow-up of 18.7 months at data base cut (4th November 2020), PFS24 rate was 48.0% for the total cohort, 65.5% for pts ≤75 years and 40.1% for pts >75 years. The median PFS was 22.9 months for all pts, 31.5 months for pts ≤75 years and 19.3 months for pts >75 years. The ORR was 59.1% in all pts, 70.8% in pts ≤75 years and 54.3% in pts >75 years. In total, 98.2% of pts developed adverse events (AEs). Grade 3/4 AEs were detected in 63.1% of pts, of which the most common were anemia (8.3%), thrombocytopenia (4.8%), dyspnea (4.8%) and pneumonia (4.8%).

Conclusion
Although the FIRST-NIS represents an elderly RW pts population with approximately 70% of pts aged >75 years, the results revealed a favorable risk-benefit profile for Rd in NDMM pts. In elderly pts >75 years, however, PFS results were lower compared to younger pts ≤75 years. Reasons might be the higher rates of advanced-stage disease and renal impairment observed in the elderly pts. Overall, the Rd combination seems to be a reasonable and orally available treatment option also for elderly pts with NDMM in the RW setting.
Keyword(s): Dexamethasone, Immunomodulatory thalidomide analog, Multiple myeloma


