
Contributions
Abstract: EP977
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Triple-class exposed relapsed/refractory multiple myeloma (RRMM) patients (ie, those exposed to a proteasome inhibitor [PI], immunomodulatory drug [IMiD], and an anti-CD38 monoclonal antibody) tend to relapse, leading to progressively worse outcomes, including shorter progression-free survival (PFS) with each subsequent relapse. CARTITUDE-1 (NCT03548207) is a single-arm, phase 1b/2 study evaluating the efficacy and safety of cilta-cel, a chimeric antigen receptor T-cell therapy with 2 B-cell maturation antigen-targeting single-domain antibodies, in heavily pretreated patients with RRMM. The CARTITUDE-1 study enrolled patients who received ≥3 prior lines of therapy [LOT] or were double refractory to a PI and IMiD, received a PI, IMiD, and anti-CD38 antibody, had an Eastern Cooperative Oncology Group performance status score of ≤1, and documented disease progression ≤12 months after the last LOT.
Aims
To compare efficacy outcomes in triple-class exposed RRMM patients treated with cilta-cel from CARTITUDE-1 (N=97) versus standard of care (SOC) in a cohort of patients who participated in global daratumumab clinical trials.
Methods
Triple-class exposed patients with RRMM who met CARTITUDE-1 eligibility criteria were identified from the follow-up data from 3 global trials of the anti-CD38 monoclonal antibody, daratumumab: POLLUX (phase 3 study of daratumumab-lenalidomide-dexamethasone vs lenalidomide-dexamethasone; NCT02076009), CASTOR (phase 3 study of daratumumab-bortezomib-dexamethasone vs bortezomib-dexamethasone; NCT02136134), and EQUULEUS (phase 1b study of daratumumab plus standard MM regimens; NCT01998971). In these trials, patients were followed for overall response, PFS, and overall survival (OS) on subsequent treatments (per physician’s choice) after discontinuation of the study treatment. These trial data were used to identify an SOC patient cohort who met CARTITUDE-1 (Sept 2020 data cutoff) eligibility criteria, including organ function. Outcomes were compared between the cilta-cel and SOC cohorts, adjusting for unbalanced prognostic baseline covariates, using inverse probability of treatment weighting (IPTW) propensity scores with logistic regression for overall response rate (ORR), and Cox models for PFS and OS to estimate the average treatment effect in the CARTITUDE-1 population. Sensitivity analyses were performed using multivariate Cox regression models and propensity score matching.
Results
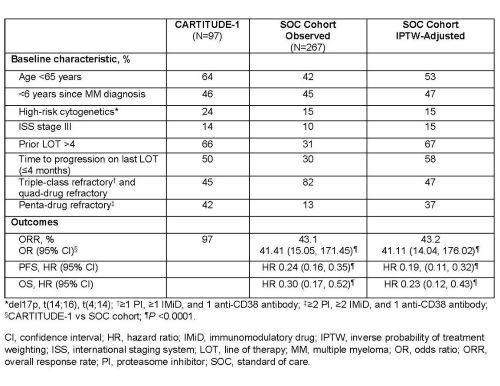
In all, 267 triple-class exposed patients with RRMM from the European Union (70.0%), North America (14.2%), Asia/Pacific (12.7%), and other regions (3.0%) met the inclusion criteria. Baseline characteristics were similar between the 2 cohorts after propensity score weighting (Table). Treatment regimens in the SOC cohort primarily included pomalidomide (26%), cyclophosphamide (26%), lenalidomide (20%), bortezomib (20%), carfilzomib (17%), and thalidomide (10%). Patients had significantly improved ORR, PFS, and OS with cilta-cel (N=97) versus SOC (N=267) (table). Cilta-cel treatment benefit was robust across the sensitivity analyses.
Conclusion
Cilta-cel shows significantly better efficacy outcomes over SOC in patients previously treated with a PI, IMiD, and daratumumab, highlighting its potential as an effective treatment option in triple-class exposed patients with RRMM.
Keyword(s): B-cell maturation antigen, CAR-T, CD38, Multiple myeloma
Abstract: EP977
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Triple-class exposed relapsed/refractory multiple myeloma (RRMM) patients (ie, those exposed to a proteasome inhibitor [PI], immunomodulatory drug [IMiD], and an anti-CD38 monoclonal antibody) tend to relapse, leading to progressively worse outcomes, including shorter progression-free survival (PFS) with each subsequent relapse. CARTITUDE-1 (NCT03548207) is a single-arm, phase 1b/2 study evaluating the efficacy and safety of cilta-cel, a chimeric antigen receptor T-cell therapy with 2 B-cell maturation antigen-targeting single-domain antibodies, in heavily pretreated patients with RRMM. The CARTITUDE-1 study enrolled patients who received ≥3 prior lines of therapy [LOT] or were double refractory to a PI and IMiD, received a PI, IMiD, and anti-CD38 antibody, had an Eastern Cooperative Oncology Group performance status score of ≤1, and documented disease progression ≤12 months after the last LOT.
Aims
To compare efficacy outcomes in triple-class exposed RRMM patients treated with cilta-cel from CARTITUDE-1 (N=97) versus standard of care (SOC) in a cohort of patients who participated in global daratumumab clinical trials.
Methods
Triple-class exposed patients with RRMM who met CARTITUDE-1 eligibility criteria were identified from the follow-up data from 3 global trials of the anti-CD38 monoclonal antibody, daratumumab: POLLUX (phase 3 study of daratumumab-lenalidomide-dexamethasone vs lenalidomide-dexamethasone; NCT02076009), CASTOR (phase 3 study of daratumumab-bortezomib-dexamethasone vs bortezomib-dexamethasone; NCT02136134), and EQUULEUS (phase 1b study of daratumumab plus standard MM regimens; NCT01998971). In these trials, patients were followed for overall response, PFS, and overall survival (OS) on subsequent treatments (per physician’s choice) after discontinuation of the study treatment. These trial data were used to identify an SOC patient cohort who met CARTITUDE-1 (Sept 2020 data cutoff) eligibility criteria, including organ function. Outcomes were compared between the cilta-cel and SOC cohorts, adjusting for unbalanced prognostic baseline covariates, using inverse probability of treatment weighting (IPTW) propensity scores with logistic regression for overall response rate (ORR), and Cox models for PFS and OS to estimate the average treatment effect in the CARTITUDE-1 population. Sensitivity analyses were performed using multivariate Cox regression models and propensity score matching.
Results
In all, 267 triple-class exposed patients with RRMM from the European Union (70.0%), North America (14.2%), Asia/Pacific (12.7%), and other regions (3.0%) met the inclusion criteria. Baseline characteristics were similar between the 2 cohorts after propensity score weighting (Table). Treatment regimens in the SOC cohort primarily included pomalidomide (26%), cyclophosphamide (26%), lenalidomide (20%), bortezomib (20%), carfilzomib (17%), and thalidomide (10%). Patients had significantly improved ORR, PFS, and OS with cilta-cel (N=97) versus SOC (N=267) (table). Cilta-cel treatment benefit was robust across the sensitivity analyses.

Conclusion
Cilta-cel shows significantly better efficacy outcomes over SOC in patients previously treated with a PI, IMiD, and daratumumab, highlighting its potential as an effective treatment option in triple-class exposed patients with RRMM.
Keyword(s): B-cell maturation antigen, CAR-T, CD38, Multiple myeloma


