Contributions
Abstract: EP975
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Panobinostat (Pano), a pan-histone deacetylase inhibitor, is approved for the treatment of relapsed or relapsed/refractory multiple myleoma (RRMM) in combination with BTZ and dex (FVd) in patients who received ≥2 prior lines of therapy, including BTZ and an IMiD. The pivotal phase 3 PANORAMA 1 trial, which used IV BTZ, demonstrated significant PFS benefit with FVd compared with placebo-Vd; however, AEs were more frequent. The randomized phase 2 PANORAMA 3 study was conducted to optimize FVd by comparing three varying regimens and by incorporating SC BTZ. This study demonstrated that 20 mg TIW provides favorable outcomes, more durable responses, and a favorable AE profile compared to those seen in PANORAMA 1. Here we present data indicating that response outcomes and AE rates in the subset of patients >75 in PANORAMA 3 were similar to those of the general trial population and to those of patients <75 years of age.
Aims
To evaluate the overall response rate (ORR; IMWG 2011 criteria) after up to 8 treatment cycles by Independent Review Committee assessment. Secondary endpoints included in this abstract are duration of response (DOR) and safety.
Methods
PANORAMA 3 (NCT02654990) was a randomized, open-label, international, multicenter phase 2 study. Eligible patients were ≥18 years old with 1‒4 prior lines of therapy, including an IMiD. Patients primarily refractory to BTZ were excluded. Patients were randomized 1:1:1 to Pano 20 mg TIW (d 1, 3, 5, 8, 10, 12), Pano 20 mg BIW (d 1, 4, 8, 11), or Pano 10 mg TIW (d 1, 3, 5, 8, 10, 12), all administered in 21-day cycles. Randomization was stratified by age at screening (≤75 vs >75 years). For Cycles 1–4, patients ≤75 years old received SC BTZ 1.3mg/m(2) BIW (d 1, 4, 8, 11) and oral dex 20mg d 1, 2, 4, 5, 8, 9, 11, 12. Patients aged <75 years from cycle 5 onwards, and patients > 75 years for all cycles, received BTZ 1.3 mg/m(2) weekly (d1 and 8) and dex 20mg on d 1, 2, 8, and 9.
Results
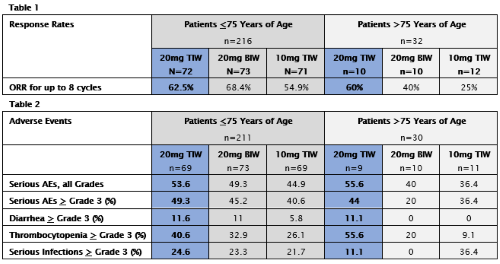
In total, 248 patients were randomized and 241 patients received treatment. Of the patients who received treatment, 211 patients were <75 years of age and 30 patients were >75 years of age. In the overall patient population, across the 3 arms, the ORR after 8 cycles was 62.2%, and the median duration of response for the 20mg TIW arm, across all age groups, was 22 months. Median DOR in patients >75 years of age for each specific treatment arm could not be calculated and will be presented subsequently. Across the general study population, treatment-related Grade ≥3 AEs were reported in 78%, 72%, and 54% of patients in the 20 TIW, 20 BIW, and 10 TIW arms respectively; serious AEs were reported in 54%, 48%, and 44% of patients respectively; and discontinuations due to AEs occurred in 29.5%, 28%, and 15% of patients respectively. See Table 1 for response rates and Table 2 for adverse events for patients <75 and >75 years of age.
Conclusion
In PANORAMA 3, SC BTZ improved the tolerability of FVd relative to that seen with IV BTZ in PANORAMA 1. Across the entire patient population, the most durable and deepest responses were observed in the 20 mg TIW arm, which yielded a median DOR of 22 months. In PANORAMA 3, the efficacy outcomes seen in the subset of patients >75 years of age were similar to those of the general study population and to those of the group <75. Importantly, in patients >75 years of age, rates of high-grade AEs, including diarrhea, were similar to those seen in the general study population and to those seen in the group <75.
Keyword(s): Elderly, HDAC inhibitor, Myeloma
Abstract: EP975
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Panobinostat (Pano), a pan-histone deacetylase inhibitor, is approved for the treatment of relapsed or relapsed/refractory multiple myleoma (RRMM) in combination with BTZ and dex (FVd) in patients who received ≥2 prior lines of therapy, including BTZ and an IMiD. The pivotal phase 3 PANORAMA 1 trial, which used IV BTZ, demonstrated significant PFS benefit with FVd compared with placebo-Vd; however, AEs were more frequent. The randomized phase 2 PANORAMA 3 study was conducted to optimize FVd by comparing three varying regimens and by incorporating SC BTZ. This study demonstrated that 20 mg TIW provides favorable outcomes, more durable responses, and a favorable AE profile compared to those seen in PANORAMA 1. Here we present data indicating that response outcomes and AE rates in the subset of patients >75 in PANORAMA 3 were similar to those of the general trial population and to those of patients <75 years of age.
Aims
To evaluate the overall response rate (ORR; IMWG 2011 criteria) after up to 8 treatment cycles by Independent Review Committee assessment. Secondary endpoints included in this abstract are duration of response (DOR) and safety.
Methods
PANORAMA 3 (NCT02654990) was a randomized, open-label, international, multicenter phase 2 study. Eligible patients were ≥18 years old with 1‒4 prior lines of therapy, including an IMiD. Patients primarily refractory to BTZ were excluded. Patients were randomized 1:1:1 to Pano 20 mg TIW (d 1, 3, 5, 8, 10, 12), Pano 20 mg BIW (d 1, 4, 8, 11), or Pano 10 mg TIW (d 1, 3, 5, 8, 10, 12), all administered in 21-day cycles. Randomization was stratified by age at screening (≤75 vs >75 years). For Cycles 1–4, patients ≤75 years old received SC BTZ 1.3mg/m(2) BIW (d 1, 4, 8, 11) and oral dex 20mg d 1, 2, 4, 5, 8, 9, 11, 12. Patients aged <75 years from cycle 5 onwards, and patients > 75 years for all cycles, received BTZ 1.3 mg/m(2) weekly (d1 and 8) and dex 20mg on d 1, 2, 8, and 9.
Results
In total, 248 patients were randomized and 241 patients received treatment. Of the patients who received treatment, 211 patients were <75 years of age and 30 patients were >75 years of age. In the overall patient population, across the 3 arms, the ORR after 8 cycles was 62.2%, and the median duration of response for the 20mg TIW arm, across all age groups, was 22 months. Median DOR in patients >75 years of age for each specific treatment arm could not be calculated and will be presented subsequently. Across the general study population, treatment-related Grade ≥3 AEs were reported in 78%, 72%, and 54% of patients in the 20 TIW, 20 BIW, and 10 TIW arms respectively; serious AEs were reported in 54%, 48%, and 44% of patients respectively; and discontinuations due to AEs occurred in 29.5%, 28%, and 15% of patients respectively. See Table 1 for response rates and Table 2 for adverse events for patients <75 and >75 years of age.
Conclusion
In PANORAMA 3, SC BTZ improved the tolerability of FVd relative to that seen with IV BTZ in PANORAMA 1. Across the entire patient population, the most durable and deepest responses were observed in the 20 mg TIW arm, which yielded a median DOR of 22 months. In PANORAMA 3, the efficacy outcomes seen in the subset of patients >75 years of age were similar to those of the general study population and to those of the group <75. Importantly, in patients >75 years of age, rates of high-grade AEs, including diarrhea, were similar to those seen in the general study population and to those seen in the group <75.
Keyword(s): Elderly, HDAC inhibitor, Myeloma