
Contributions
Abstract: EP969
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
The safety and efficacy of daratumumab (DARA) plus cyclophosphamide, bortezomib, and dexamethasone (CyBorD) as an immunomodulatory drug-sparing regimen in newly-diagnosed patients with multiple myeloma (NDMM) and relapsed MM (RMM) were demonstrated in a primary analysis of LYRA, a phase 2, single-arm, community practice-based study (NCT02951819). An updated analysis of the study further showed that responses deepened with DARA maintenance therapy.
Aims
To report the final end-of-study analysis of LYRA.
Methods
Adult patients (aged ≥18 years) from the United States with MM (International Myeloma Working Group criteria) and no more than 1 prior line of therapy received 4–8 induction cycles of DARA plus CyBorD (oral cyclophosphamide 300 mg/m2 once weekly [QW]; subcutaneous bortezomib 1.5 mg/m2 on Days [D] 1, 8, and 15; oral or intravenous (IV) dexamethasone 40 mg QW every 28 days; DARA IV, 8 mg/kg on D1 and D2 of cycle [C] 1, 16 mg/kg QW on C1D8–C2, 16 mg/kg Q2W in C3–C6, and 16 mg/kg Q4W in C7–C8). Written informed consent was obtained from all patients. Following induction, eligible patients were allowed to receive autologous stem cell transplantation (ASCT). Patients received ≤12 maintenance cycles of DARA 16 mg/kg IV Q4W and were then followed up for ≤36 months after induction.
Results
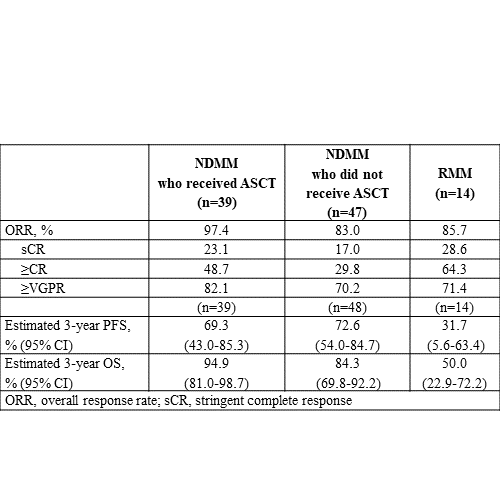
Of 101 patients (NDMM, 87; RMM, 14), 36% had high-risk cytogenetics. Patients with NDMM received a median of 6 induction cycles; those with RMM received 8. Of 87 patients with NDMM, 39 (44.8%) received ASCT and 63 (72.4%) completed 12 months of maintenance therapy. Of 14 patients with RMM, 1 (7.1%) received ASCT and 7 (50.0%) completed 12 months of maintenance therapy. A higher proportion of patients with NDMM who received ASCT achieved very good partial response or better (≥VGPR; 82.1%) and complete response or better (≥CR; 48.7%) vs patients who did not receive ASCT (70.2% and 29.8%, respectively; Table). For patients with NDMM, median progression-free survival (PFS) and overall survival (OS) were not reached (median follow up, 35.7 months). Estimated 3-year PFS rates were 69.3% for patients with NDMM who received ASCT and 72.6% for those who did not; estimated 3-year OS rates were 94.9% and 84.3%, respectively. Efficacy outcomes for patients with RMM are reported in Table. Overall, 62.0% of patients experienced grade 3/4 treatment-emergent adverse events (TEAEs), and neutropenia (14.0%) was most common (≥10%). Serious TEAEs were observed in 33.0% of patients, and pneumonia (4.0%) and pulmonary embolism (3.0%) were most common. TEAEs leading to death occurred in 2.0% of patients (all unrelated to study treatment). Nearly half of patients (56.0%) experienced infusion-related reactions; most were mild (4.0% had grade 3/4 events).
Conclusion
In patients with NDMM or RMM, induction with DARA plus CyBorD followed by DARA alone as maintenance therapy resulted in durable and deep responses, with a 3-year PFS rate of 70%, regardless of ASCT status. No new safety concerns were identified with longer follow up.
Keyword(s): Induction chemotherapy, Multiple myeloma
Abstract: EP969
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
The safety and efficacy of daratumumab (DARA) plus cyclophosphamide, bortezomib, and dexamethasone (CyBorD) as an immunomodulatory drug-sparing regimen in newly-diagnosed patients with multiple myeloma (NDMM) and relapsed MM (RMM) were demonstrated in a primary analysis of LYRA, a phase 2, single-arm, community practice-based study (NCT02951819). An updated analysis of the study further showed that responses deepened with DARA maintenance therapy.
Aims
To report the final end-of-study analysis of LYRA.
Methods
Adult patients (aged ≥18 years) from the United States with MM (International Myeloma Working Group criteria) and no more than 1 prior line of therapy received 4–8 induction cycles of DARA plus CyBorD (oral cyclophosphamide 300 mg/m2 once weekly [QW]; subcutaneous bortezomib 1.5 mg/m2 on Days [D] 1, 8, and 15; oral or intravenous (IV) dexamethasone 40 mg QW every 28 days; DARA IV, 8 mg/kg on D1 and D2 of cycle [C] 1, 16 mg/kg QW on C1D8–C2, 16 mg/kg Q2W in C3–C6, and 16 mg/kg Q4W in C7–C8). Written informed consent was obtained from all patients. Following induction, eligible patients were allowed to receive autologous stem cell transplantation (ASCT). Patients received ≤12 maintenance cycles of DARA 16 mg/kg IV Q4W and were then followed up for ≤36 months after induction.
Results
Of 101 patients (NDMM, 87; RMM, 14), 36% had high-risk cytogenetics. Patients with NDMM received a median of 6 induction cycles; those with RMM received 8. Of 87 patients with NDMM, 39 (44.8%) received ASCT and 63 (72.4%) completed 12 months of maintenance therapy. Of 14 patients with RMM, 1 (7.1%) received ASCT and 7 (50.0%) completed 12 months of maintenance therapy. A higher proportion of patients with NDMM who received ASCT achieved very good partial response or better (≥VGPR; 82.1%) and complete response or better (≥CR; 48.7%) vs patients who did not receive ASCT (70.2% and 29.8%, respectively; Table). For patients with NDMM, median progression-free survival (PFS) and overall survival (OS) were not reached (median follow up, 35.7 months). Estimated 3-year PFS rates were 69.3% for patients with NDMM who received ASCT and 72.6% for those who did not; estimated 3-year OS rates were 94.9% and 84.3%, respectively. Efficacy outcomes for patients with RMM are reported in Table. Overall, 62.0% of patients experienced grade 3/4 treatment-emergent adverse events (TEAEs), and neutropenia (14.0%) was most common (≥10%). Serious TEAEs were observed in 33.0% of patients, and pneumonia (4.0%) and pulmonary embolism (3.0%) were most common. TEAEs leading to death occurred in 2.0% of patients (all unrelated to study treatment). Nearly half of patients (56.0%) experienced infusion-related reactions; most were mild (4.0% had grade 3/4 events).

Conclusion
In patients with NDMM or RMM, induction with DARA plus CyBorD followed by DARA alone as maintenance therapy resulted in durable and deep responses, with a 3-year PFS rate of 70%, regardless of ASCT status. No new safety concerns were identified with longer follow up.
Keyword(s): Induction chemotherapy, Multiple myeloma


