
Contributions
Abstract: EP963
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Despite an increasing number of treatment options for RRMM, there remains a need for efficacious & tolerable therapies for pts who are refractory to treatment or continue to relapse. Proteasome inhibitors (PIs) are a backbone of therapy, but parenteral PIs may be limited by treatment burden & the need for clinic/hospital visits. The first oral PI, ixazomib, is approved in combination with Rd (IRd) for the treatment of MM pts who have received ≥1 prior lines of therapy. Approval was based on the double-blind, placebo-controlled, phase 3 TOURMALINE‑MM1 study (Moreau NEJM 2016), which demonstrated a statistically significant improvement in progression-free survival (PFS) with IRd vs pRd (median PFS 20.6 vs 14.7 mo, HR 0.74, p=0.01). With limited additional toxicity vs pRd, all-oral IRd is an important option for RRMM pts.
Aims
To report final analyses for overall survival (OS) in the intent-to-treat (ITT) population (a key secondary endpoint) & subgroups of interest.
Methods
Randomization to IRd (n=360) or pRd (n=362) was stratified by number of prior therapies (1 vs 2/3), PI exposure, & International Staging System (ISS) disease stage (I/II vs III). Treatment continued until disease progression/unacceptable toxicity.
Results
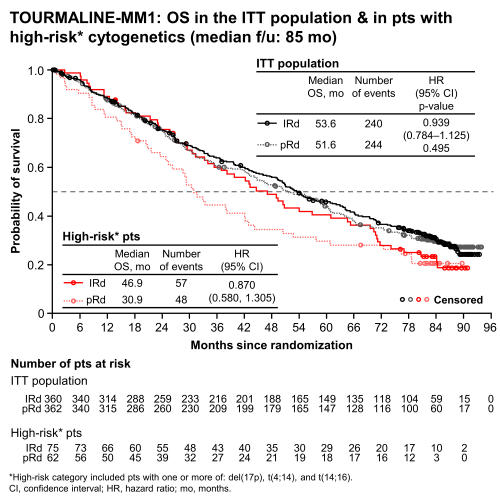
At data cutoff (9/28/2020), median follow-up (f/u) was ~7 y (85 mo). Pts had received a median of 18 (range 1–99) & 16 (1–100) cycles of IRd & pRd, respectively. Overall, 32% of pts were alive at last OS f/u; 4% in each arm were still on treatment. Median OS with IRd vs pRd (ITT population) was 53.6 vs 51.6 mo (HR 0.939, p=0.495, Figure); this favorable trend was not statistically significant, but occurred in the context of the longest OS (both arms) reported to date in phase 3 Rd studies in RRMM. OS benefit with IRd vs pRd (lower HRs) was seen in predefined subgroups with adverse-risk characteristics including: refractory to any (HR 0.794) or last (HR 0.742) treatment line; age >65–75 y (HR 0.757); ISS stage III (HR 0.779); 2/3 prior therapies (HR 0.845); high-risk cytogenetics [≥1 of del(17p), t(4:14), t(14;16) (HR 0.870, Figure), & 1q21 amplification (HR 0.862)]. OS benefit was also seen in pts with standard-risk cytogenetics (HR 0.875). Imbalances in subsequent therapy in both arms may have led to inconsistencies in OS benefit for IRd pts. While 72% of IRd & 70% of pRd pts received ≥1 subsequent anticancer therapy, pRd pts began this therapy earlier than IRd pts. Of IRd vs pRd pts receiving ≥1 subsequent therapy, 25 vs 34% received daratumumab, 72 vs 77% received PIs (57 vs 62% bortezomib, 27 vs 33% carfilzomib), & 4 vs 10% had an autologous stem cell transplant. Most pts (IRd 92%, pRd 85%) remained blinded at time of next treatment, and while similar proportions of blinded pts received a PI or non-PI next-line therapy in both arms, unblinding strongly influenced next-line therapy decisions. OS benefit was reduced in pts who received a PI immediately after progression on IRd vs pts who received a PI after pRd. No new/additional safety concerns were evident during the 7-y f/u period, & the rate of new primary malignancies was similar in both arms (IRd 10%, pRd 12%).
Conclusion
At this final analysis of TOURMALINE-MM1, median OS with IRd & pRd were the longest reported to date in phase 3 Rd studies in RRMM. Although OS benefit with IRd vs pRd was not statistically significant in the ITT population, greater OS benefit was seen in subgroups with adverse prognostic factors, including high-risk cytogenetics. OS interpretation was confounded by imbalances in subsequent therapies, including extensive use of PIs & novel monoclonal antibodies.
Keyword(s): Clinical trial, Long-term follow-up, Multiple myeloma, Proteasome inhibitor
Abstract: EP963
Type: E-Poster Presentation
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Despite an increasing number of treatment options for RRMM, there remains a need for efficacious & tolerable therapies for pts who are refractory to treatment or continue to relapse. Proteasome inhibitors (PIs) are a backbone of therapy, but parenteral PIs may be limited by treatment burden & the need for clinic/hospital visits. The first oral PI, ixazomib, is approved in combination with Rd (IRd) for the treatment of MM pts who have received ≥1 prior lines of therapy. Approval was based on the double-blind, placebo-controlled, phase 3 TOURMALINE‑MM1 study (Moreau NEJM 2016), which demonstrated a statistically significant improvement in progression-free survival (PFS) with IRd vs pRd (median PFS 20.6 vs 14.7 mo, HR 0.74, p=0.01). With limited additional toxicity vs pRd, all-oral IRd is an important option for RRMM pts.
Aims
To report final analyses for overall survival (OS) in the intent-to-treat (ITT) population (a key secondary endpoint) & subgroups of interest.
Methods
Randomization to IRd (n=360) or pRd (n=362) was stratified by number of prior therapies (1 vs 2/3), PI exposure, & International Staging System (ISS) disease stage (I/II vs III). Treatment continued until disease progression/unacceptable toxicity.
Results
At data cutoff (9/28/2020), median follow-up (f/u) was ~7 y (85 mo). Pts had received a median of 18 (range 1–99) & 16 (1–100) cycles of IRd & pRd, respectively. Overall, 32% of pts were alive at last OS f/u; 4% in each arm were still on treatment. Median OS with IRd vs pRd (ITT population) was 53.6 vs 51.6 mo (HR 0.939, p=0.495, Figure); this favorable trend was not statistically significant, but occurred in the context of the longest OS (both arms) reported to date in phase 3 Rd studies in RRMM. OS benefit with IRd vs pRd (lower HRs) was seen in predefined subgroups with adverse-risk characteristics including: refractory to any (HR 0.794) or last (HR 0.742) treatment line; age >65–75 y (HR 0.757); ISS stage III (HR 0.779); 2/3 prior therapies (HR 0.845); high-risk cytogenetics [≥1 of del(17p), t(4:14), t(14;16) (HR 0.870, Figure), & 1q21 amplification (HR 0.862)]. OS benefit was also seen in pts with standard-risk cytogenetics (HR 0.875). Imbalances in subsequent therapy in both arms may have led to inconsistencies in OS benefit for IRd pts. While 72% of IRd & 70% of pRd pts received ≥1 subsequent anticancer therapy, pRd pts began this therapy earlier than IRd pts. Of IRd vs pRd pts receiving ≥1 subsequent therapy, 25 vs 34% received daratumumab, 72 vs 77% received PIs (57 vs 62% bortezomib, 27 vs 33% carfilzomib), & 4 vs 10% had an autologous stem cell transplant. Most pts (IRd 92%, pRd 85%) remained blinded at time of next treatment, and while similar proportions of blinded pts received a PI or non-PI next-line therapy in both arms, unblinding strongly influenced next-line therapy decisions. OS benefit was reduced in pts who received a PI immediately after progression on IRd vs pts who received a PI after pRd. No new/additional safety concerns were evident during the 7-y f/u period, & the rate of new primary malignancies was similar in both arms (IRd 10%, pRd 12%).

Conclusion
At this final analysis of TOURMALINE-MM1, median OS with IRd & pRd were the longest reported to date in phase 3 Rd studies in RRMM. Although OS benefit with IRd vs pRd was not statistically significant in the ITT population, greater OS benefit was seen in subgroups with adverse prognostic factors, including high-risk cytogenetics. OS interpretation was confounded by imbalances in subsequent therapies, including extensive use of PIs & novel monoclonal antibodies.
Keyword(s): Clinical trial, Long-term follow-up, Multiple myeloma, Proteasome inhibitor


