Contributions
Abstract: EP926
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
Venetoclax (Ven) is a selective, potent BCL-2 inhibitor. Ven + azacitidine (Aza) were associated with a combined complete remission (CR)/marrow CR (mCR) rate of 79% in a phase 1b study of patients (pts) with HR-MDS (NCT02942290).
Aims
We compared two different dose modification strategies to manage expected hematologic toxicities in two safety expansion cohorts with similar follow-up periods.
Methods
Pts (≥18 yrs) diagnosed with treatment-naïve IPSS intermediate-2 or high-risk MDS with ECOG ≤2 were enrolled. Aza 75 mg/m2 (i.v. or sub-Q, daily) was administered for 7 days (d) and Ven was administered at 400 mg for 14d in each 28d cycle. In both cohorts, dose modification during Cycle 1 was not recommended; dose modifications in subsequent cycles were prescribed for adverse events. In Safety Expansion Cohort 1 (SE1), either Aza or Ven were initially reduced according to investigator’s choice for significant neutrophil or platelet toxicity. Dose reductions per protocol were 33% for Aza and 50% for Ven (for 14d each cycle). In subsequent cycles, Ven duration could be shortened to 9d of each cycle. In Safety Expansion Cohort 2 (SE2), dose modification guidelines recommended stepwise reductions, first in Aza dose (first to 50 mg/m2, then 36 mg/m2) and subsequently in Ven duration to 7d of each cycle (Ven 400 mg). The impact of each dose modification strategy on safety and efficacy in SE1 vs SE2 was compared. Worsening of treatment-emergent adverse events (TEAE) grades from baseline (BL) was analyzed by cycle. Responses were evaluated using IWG 2006 criteria. Analyses included all pts who received ≥1 dose of study drug.
Results
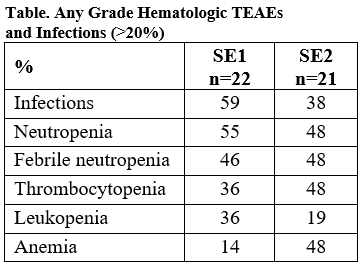
We compared 22 pts in SE1 and 21 pts in SE2 with median (range) follow-up of 7.5 (1.0–8.9) and 7.9 (1.8–10.1) mos, respectively. A similar frequency of ≥G3 hematologic TEAEs (approximate %) were reported in SE1 and SE2, respectively, including anemia (14% and 33%), febrile neutropenia (46% and 48%), leukopenia (36% and 19%), neutropenia (55% and 48%) and thrombocytopenia (32% and 38%). Infections (59% and 38%) were more frequent in SE1 than SE2. In a longitudinal analysis, there were more TEAE grade increases from BL to Cycle 1 in SE2 vs SE1. This could be as accounted by pts in SE1 and SE2 having unbalanced susceptibility to AEs at BL, as SE1 and SE2 pts received identical Aza + Ven doses in Cycle 1. Response rates were identical: 86% of pts in both SE1 and SE2 had CR or mCR. For pts with mCR, hematologic improvement occurred in 50% of SE1 and 46% of SE2 pts.
Conclusion
No obvious hematologic differences were observed when reducing Aza before Ven (SE2) in MDS compared to investigator’s choice (SE1). Both approaches had a similar acceptable safety profile without compromising efficacy for pts with HR-MDS.
Keyword(s): BCL2, Clinical trial, Hematological malignancy, Myelodysplasia
Abstract: EP926
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
Venetoclax (Ven) is a selective, potent BCL-2 inhibitor. Ven + azacitidine (Aza) were associated with a combined complete remission (CR)/marrow CR (mCR) rate of 79% in a phase 1b study of patients (pts) with HR-MDS (NCT02942290).
Aims
We compared two different dose modification strategies to manage expected hematologic toxicities in two safety expansion cohorts with similar follow-up periods.
Methods
Pts (≥18 yrs) diagnosed with treatment-naïve IPSS intermediate-2 or high-risk MDS with ECOG ≤2 were enrolled. Aza 75 mg/m2 (i.v. or sub-Q, daily) was administered for 7 days (d) and Ven was administered at 400 mg for 14d in each 28d cycle. In both cohorts, dose modification during Cycle 1 was not recommended; dose modifications in subsequent cycles were prescribed for adverse events. In Safety Expansion Cohort 1 (SE1), either Aza or Ven were initially reduced according to investigator’s choice for significant neutrophil or platelet toxicity. Dose reductions per protocol were 33% for Aza and 50% for Ven (for 14d each cycle). In subsequent cycles, Ven duration could be shortened to 9d of each cycle. In Safety Expansion Cohort 2 (SE2), dose modification guidelines recommended stepwise reductions, first in Aza dose (first to 50 mg/m2, then 36 mg/m2) and subsequently in Ven duration to 7d of each cycle (Ven 400 mg). The impact of each dose modification strategy on safety and efficacy in SE1 vs SE2 was compared. Worsening of treatment-emergent adverse events (TEAE) grades from baseline (BL) was analyzed by cycle. Responses were evaluated using IWG 2006 criteria. Analyses included all pts who received ≥1 dose of study drug.
Results
We compared 22 pts in SE1 and 21 pts in SE2 with median (range) follow-up of 7.5 (1.0–8.9) and 7.9 (1.8–10.1) mos, respectively. A similar frequency of ≥G3 hematologic TEAEs (approximate %) were reported in SE1 and SE2, respectively, including anemia (14% and 33%), febrile neutropenia (46% and 48%), leukopenia (36% and 19%), neutropenia (55% and 48%) and thrombocytopenia (32% and 38%). Infections (59% and 38%) were more frequent in SE1 than SE2. In a longitudinal analysis, there were more TEAE grade increases from BL to Cycle 1 in SE2 vs SE1. This could be as accounted by pts in SE1 and SE2 having unbalanced susceptibility to AEs at BL, as SE1 and SE2 pts received identical Aza + Ven doses in Cycle 1. Response rates were identical: 86% of pts in both SE1 and SE2 had CR or mCR. For pts with mCR, hematologic improvement occurred in 50% of SE1 and 46% of SE2 pts.
Conclusion
No obvious hematologic differences were observed when reducing Aza before Ven (SE2) in MDS compared to investigator’s choice (SE1). Both approaches had a similar acceptable safety profile without compromising efficacy for pts with HR-MDS.
Keyword(s): BCL2, Clinical trial, Hematological malignancy, Myelodysplasia