Contributions
Abstract: EP925
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
Treatment response evaluation in MDS relies on serial assessment of peripheral blood (PB) counts, bone marrow (BM) morphology, and cytogenetics (IWG2006; Cheson et al, Blood 2006). BM sampling is invasive, whereas biomarkers of response measured in PB could provide a less invasive alternative for monitoring treatment responses. Most MDS and CMML patients carry somatic driver mutations, that can be detected in PB. Consistent reductions in PB variant allele frequencies (VAFs) have been detected during decitabine therapy for MDS (Duncavage et al, Blood 2017), suggesting that detection of clonal dynamics in PB may be used as an early predictor of therapy response.
Aims
We evaluated whether changes in mutational load, using high sensitivity mutation-specific droplet digital PCR (ddPCR) of PB samples, could be used as an early sign of treatment response in MDS/CMML.
Methods
Patients were recruited between 1/2016-5/2018 at Helsinki University Hospital. All patients signed an informed consent. Serial PB samples were collected before treatment and during routine visits. Pre-treatment samples were analyzed with an in-house NGS myeloid panel of 45 genes. Mutation-specific ddPCR were designed, and on-treatment samples were analyzed with the QX200 Droplet Digital PCR system.
Results
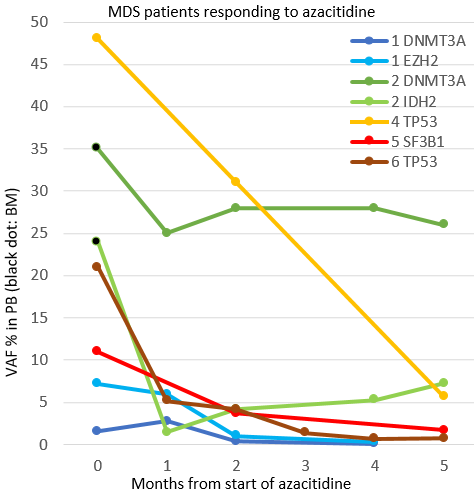
The results of the first ten patients are reported here. Seven MDS patients were treated with azacitidine (AZA), one with lenalidomide (LEN), one CMML patient was treated with hydroxyurea (HU) and one with both HU and AZA. Median age at treatment initiation was 68 years (range 48-81). The median follow-up was 11 months (range 4-33). Altogether 17 myeloid driver mutations were found in the NGS and 15 of these (4 SF3B1, 2 DNMT3A, 2 TP53, 2 IDH2, 2 EZH2, 2 NRAS, U2AF1) were analyzed with ddPCR at 4-13 timepoints (median 7,5/patient). For one mutation in SRFS2 there was not an available probe. For a mutation in NF1 the analyses are pending. Using IWG2006 response criteria, seven patients responded to treatment (two CR, three mCR, two HI) and three patients had stable disease (SD). All responding (CR, mCR, HI) MDS-patients (n=6) had a decrease in VAF with a mean relative decrease of 71 % (range 20-94) from pretreatment to samples collected after the second AZA (n=5, figure) or LEN cycle (n=1). The decrease in VAF was detected 28-251 (median 43) days before the IWG2006-response was verified. Patient 2 in the figure had a DNMT3A-mutation that decreased only slightly (35% (BM) to 25 %), whilst the IDH2 seemed to decrease rapidly after the first AZA-cycle, from 24% (BM) to 1,5 %, suggesting that the DNMT3A-mutation may reflect a pre-malignant clone. Two MDS cases with SD had mutations in SF3B1, one with stable VAF and the other with VAF-decrease (24 % to 11 %). One CMML-patient had a mCR-response at 10 months after HU and AZA; NRAS VAF decreased only from 46 % to 35 % and IDH2 from 46 % to 36 %. The second CMML case had SD and reduction in NRAS from 45 % to 33% and U2AF1 from 46 % to 32 %. Five patients lost their response during treatment. One patient in HI, had an increase from 5,7% to 20 % in TP53 55 days before progression. One patient in CR had an increase from 0,12 % to 25 % in SF3B1 49 days before progression. Another CR patient had an increase in TP53 from 1,4 % to 4,9 % at progression. Two SD patients had moderate VAF-increases at progression; one in SF3B1 from 18 % to 34 %, another in NRAS from 33 % to 43 % and U2AF1 from 32 % to 42 %.
Conclusion
Detection of peripheral blood clonal dynamics using ddPCR may serve as a non-invasive tool for early response evaluation during azacitidine treatment in MDS.
Keyword(s): Azacitidine, MDS, PCR, Treatment
Abstract: EP925
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
Treatment response evaluation in MDS relies on serial assessment of peripheral blood (PB) counts, bone marrow (BM) morphology, and cytogenetics (IWG2006; Cheson et al, Blood 2006). BM sampling is invasive, whereas biomarkers of response measured in PB could provide a less invasive alternative for monitoring treatment responses. Most MDS and CMML patients carry somatic driver mutations, that can be detected in PB. Consistent reductions in PB variant allele frequencies (VAFs) have been detected during decitabine therapy for MDS (Duncavage et al, Blood 2017), suggesting that detection of clonal dynamics in PB may be used as an early predictor of therapy response.
Aims
We evaluated whether changes in mutational load, using high sensitivity mutation-specific droplet digital PCR (ddPCR) of PB samples, could be used as an early sign of treatment response in MDS/CMML.
Methods
Patients were recruited between 1/2016-5/2018 at Helsinki University Hospital. All patients signed an informed consent. Serial PB samples were collected before treatment and during routine visits. Pre-treatment samples were analyzed with an in-house NGS myeloid panel of 45 genes. Mutation-specific ddPCR were designed, and on-treatment samples were analyzed with the QX200 Droplet Digital PCR system.
Results
The results of the first ten patients are reported here. Seven MDS patients were treated with azacitidine (AZA), one with lenalidomide (LEN), one CMML patient was treated with hydroxyurea (HU) and one with both HU and AZA. Median age at treatment initiation was 68 years (range 48-81). The median follow-up was 11 months (range 4-33). Altogether 17 myeloid driver mutations were found in the NGS and 15 of these (4 SF3B1, 2 DNMT3A, 2 TP53, 2 IDH2, 2 EZH2, 2 NRAS, U2AF1) were analyzed with ddPCR at 4-13 timepoints (median 7,5/patient). For one mutation in SRFS2 there was not an available probe. For a mutation in NF1 the analyses are pending. Using IWG2006 response criteria, seven patients responded to treatment (two CR, three mCR, two HI) and three patients had stable disease (SD). All responding (CR, mCR, HI) MDS-patients (n=6) had a decrease in VAF with a mean relative decrease of 71 % (range 20-94) from pretreatment to samples collected after the second AZA (n=5, figure) or LEN cycle (n=1). The decrease in VAF was detected 28-251 (median 43) days before the IWG2006-response was verified. Patient 2 in the figure had a DNMT3A-mutation that decreased only slightly (35% (BM) to 25 %), whilst the IDH2 seemed to decrease rapidly after the first AZA-cycle, from 24% (BM) to 1,5 %, suggesting that the DNMT3A-mutation may reflect a pre-malignant clone. Two MDS cases with SD had mutations in SF3B1, one with stable VAF and the other with VAF-decrease (24 % to 11 %). One CMML-patient had a mCR-response at 10 months after HU and AZA; NRAS VAF decreased only from 46 % to 35 % and IDH2 from 46 % to 36 %. The second CMML case had SD and reduction in NRAS from 45 % to 33% and U2AF1 from 46 % to 32 %. Five patients lost their response during treatment. One patient in HI, had an increase from 5,7% to 20 % in TP53 55 days before progression. One patient in CR had an increase from 0,12 % to 25 % in SF3B1 49 days before progression. Another CR patient had an increase in TP53 from 1,4 % to 4,9 % at progression. Two SD patients had moderate VAF-increases at progression; one in SF3B1 from 18 % to 34 %, another in NRAS from 33 % to 43 % and U2AF1 from 32 % to 42 %.
Conclusion
Detection of peripheral blood clonal dynamics using ddPCR may serve as a non-invasive tool for early response evaluation during azacitidine treatment in MDS.
Keyword(s): Azacitidine, MDS, PCR, Treatment