
Contributions
Abstract: EP920
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
MEDALIST (NCT02631070) is an ongoing, randomized, phase 3 trial evaluating the efficacy and safety of luspatercept, the first and only erythroid maturation agent, which reduced red blood cell (RBC) transfusion burden (TB) and led to significant rates of transfusion independence (TI) in patients (pts) with anemia due to lower-risk myelodysplastic syndromes (LR-MDS) with ring sideroblasts (Fenaux P, et al. N Engl J Med 2020;382:140–151). TI is associated with improved prognosis in pts with MDS. The effect of luspatercept treatment on red blood cell transfusion (RBCT) units and visits in pts with different baseline TB warrants further investigation.
Aims
To evaluate the effect of luspatercept on the cumulative number of RBCT units and visits in pts with LR-MDS according to their baseline TB levels in the MEDALIST study.
Methods
Eligible pts were ≥18 years old; were refractory, intolerant, or unlikely to respond to erythropoiesis-stimulating agents; and required regular RBC transfusions (≥2 units/8 weeks [wks]) in the 16 wks prior to randomization. Overall, 229 pts were randomized 2:1 to receive either luspatercept (n=153) or placebo (n=76) every 3 wks through the clinical assessment visit at Wk 25, defined as 24 calendar wks after the first dose, regardless of dose delays. Pts experiencing clinical benefit continued treatment beyond 24 wks. The data cutoff for this analysis was July 1, 2019. Cumulative mean number of RBCT units and visits were estimated using the Nelson-Aalen nonparametric estimator with robust variance estimate for each treatment group. Luspatercept responders were defined as pts achieving RBC-TI for ≥8 wks during wks 1–24. At baseline, pts with <6 RBCT units/8 wks were defined as low TB (LTB) and pts with ≥6 RBCT units/8 wks were defined as high TB (HTB).
Results
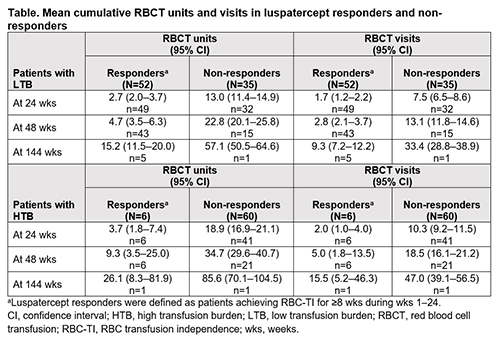
Similar proportions of pts in the luspatercept and placebo groups were LTB and HTB at baseline; 56.9% (n=87/153) in luspatercept vs 56.6% (n=43/76) in placebo were LTB, whereas 43.1% (n=66/153) vs 43.4% (n=33/76) were HTB, respectively. Regardless of baseline TB, pts receiving luspatercept had lower mean cumulative RBCT units and visits at 24 wks relative to placebo. Mean cumulative RBCT units (95% confidence interval [CI]) in LTB pts was 6.8 (5.6–8.4) in the luspatercept group (n=81/87) vs 13.2 (11.5–15.2) in the placebo group (n=38/43); and for HTB pts, was 17.2 (15.1–19.6) in the luspatercept group (n=47/66) vs 24.2 (21.3–27.4) in the placebo group (n=30/33). Mean cumulative RBCT visits (95% CI) in LTB pts was 4.0 (3.3–4.8) in the luspatercept group (n=81/87) vs 7.2 (6.3–8.3) in the placebo group (n=38/43); and for HTB pts, was 9.4 (8.3–10.7) in the luspatercept group (n=47/66) vs 12.5 (11.0–14.2) in the placebo group (n=30/33). Luspatercept responders continued to show additional reductions in RBCT units and visits beyond 24 wks through 144 wks, relative to non-responders in both LTB and HTB groups (Table).
Conclusion
In the MEDALIST trial, luspatercept reduced cumulative RBCT units and visits relative to placebo over the first 24-wk period in pts with LR-MDS regardless of their baseline TB level. Luspatercept responders showed additional benefits in RBCT unit and visit reduction relative to non-responders beyond 24 wks.
Keyword(s): Clinical trial, MDS, Phase III, Transfusion
Abstract: EP920
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
MEDALIST (NCT02631070) is an ongoing, randomized, phase 3 trial evaluating the efficacy and safety of luspatercept, the first and only erythroid maturation agent, which reduced red blood cell (RBC) transfusion burden (TB) and led to significant rates of transfusion independence (TI) in patients (pts) with anemia due to lower-risk myelodysplastic syndromes (LR-MDS) with ring sideroblasts (Fenaux P, et al. N Engl J Med 2020;382:140–151). TI is associated with improved prognosis in pts with MDS. The effect of luspatercept treatment on red blood cell transfusion (RBCT) units and visits in pts with different baseline TB warrants further investigation.
Aims
To evaluate the effect of luspatercept on the cumulative number of RBCT units and visits in pts with LR-MDS according to their baseline TB levels in the MEDALIST study.
Methods
Eligible pts were ≥18 years old; were refractory, intolerant, or unlikely to respond to erythropoiesis-stimulating agents; and required regular RBC transfusions (≥2 units/8 weeks [wks]) in the 16 wks prior to randomization. Overall, 229 pts were randomized 2:1 to receive either luspatercept (n=153) or placebo (n=76) every 3 wks through the clinical assessment visit at Wk 25, defined as 24 calendar wks after the first dose, regardless of dose delays. Pts experiencing clinical benefit continued treatment beyond 24 wks. The data cutoff for this analysis was July 1, 2019. Cumulative mean number of RBCT units and visits were estimated using the Nelson-Aalen nonparametric estimator with robust variance estimate for each treatment group. Luspatercept responders were defined as pts achieving RBC-TI for ≥8 wks during wks 1–24. At baseline, pts with <6 RBCT units/8 wks were defined as low TB (LTB) and pts with ≥6 RBCT units/8 wks were defined as high TB (HTB).
Results
Similar proportions of pts in the luspatercept and placebo groups were LTB and HTB at baseline; 56.9% (n=87/153) in luspatercept vs 56.6% (n=43/76) in placebo were LTB, whereas 43.1% (n=66/153) vs 43.4% (n=33/76) were HTB, respectively. Regardless of baseline TB, pts receiving luspatercept had lower mean cumulative RBCT units and visits at 24 wks relative to placebo. Mean cumulative RBCT units (95% confidence interval [CI]) in LTB pts was 6.8 (5.6–8.4) in the luspatercept group (n=81/87) vs 13.2 (11.5–15.2) in the placebo group (n=38/43); and for HTB pts, was 17.2 (15.1–19.6) in the luspatercept group (n=47/66) vs 24.2 (21.3–27.4) in the placebo group (n=30/33). Mean cumulative RBCT visits (95% CI) in LTB pts was 4.0 (3.3–4.8) in the luspatercept group (n=81/87) vs 7.2 (6.3–8.3) in the placebo group (n=38/43); and for HTB pts, was 9.4 (8.3–10.7) in the luspatercept group (n=47/66) vs 12.5 (11.0–14.2) in the placebo group (n=30/33). Luspatercept responders continued to show additional reductions in RBCT units and visits beyond 24 wks through 144 wks, relative to non-responders in both LTB and HTB groups (Table).

Conclusion
In the MEDALIST trial, luspatercept reduced cumulative RBCT units and visits relative to placebo over the first 24-wk period in pts with LR-MDS regardless of their baseline TB level. Luspatercept responders showed additional benefits in RBCT unit and visit reduction relative to non-responders beyond 24 wks.
Keyword(s): Clinical trial, MDS, Phase III, Transfusion


