
Contributions
Abstract: EP917
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
Hypomethylating agents (HMA) are the current standard of care for patients (pts) with higher-risk myelodysplastic syndromes (HR-MDS) who are ineligible for allogeneic hematopoietic stem cell transplantation (HSCT). However, overall response rates (ORRs) remain suboptimal in pts receiving azacitidine (Aza), and their expected median overall survival (OS) is less than 2 years. Venetoclax (Ven) is a selective, potent, oral BCL-2 inhibitor, which has demonstrated synergy with HMAs in preclinical and clinical studies of myeloid malignancies.
Aims
We report updated safety and efficacy in pts treated in an ongoing phase 1b study (NCT02942290) evaluating Ven+Aza for treatment-naïve HR-MDS.
Methods
Pts (≥18 years) with International Prognostic Scoring System (IPSS) intermediate-2 or high-risk MDS, bone marrow (BM) blasts <20% at baseline and ECOG ≤2 performance status were enrolled. Aza 75 mg/m2 was administered on Days (d) 1–7 of each 28-d cycle. Ven was initially given at a dose of 400 mg or 800 mg daily in 28-d cycles. Due to poor tolerance among pts with MDS, the study was amended to examine feasibility of several dose cohorts (100, 200 and 400 mg x 14 d each 28d cycle). Primary objectives were to assess the Ven+Aza safety profile and to establish the RP2D. Key secondary objectives included assessment of ORR and OS. Safety and efficacy analyses were carried out on all pts who received ≥1 dose of study drug and efficacy endpoints evaluated per IWG 2006 response criteria. Neutropenia included neutropenia, febrile neutropenia, neutrophil count decreased, neutropenic sepsis/infection, agranulocytosis. Thrombocytopenia included thrombocytopenia and platelet count decreased. Baseline mutational status was evaluated on BM aspirate samples using the Archer VariantPlex Myeloid panel.
Results
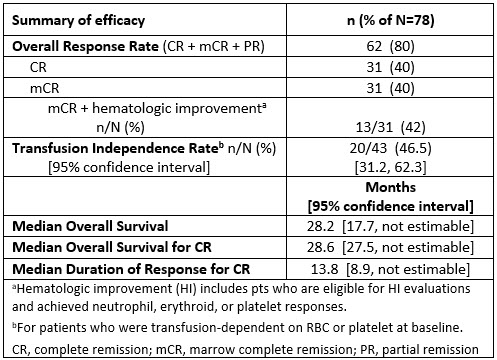
At data cutoff Dec 15, 2020, 78 pts had received Ven+Aza (51 pts received Ven at the RP2D of 400 mg x 14 d), with a median follow-up time of 23 mos (range 0.1–44.2). Median age was 70 years (range 26–87); 72% male; and 90% had excess BM blasts (>5 to ≤10%, n=21; >10 to ≤20%, n=49). Grade 3/4 neutropenia (59%) or thrombocytopenia (33%) were present at baseline. All pts experienced ≥1 treatment-emergent adverse event (TEAE), the most common being neutropenia (83%), nausea (55%), and constipation (54%). Grade 3/4 TEAEs were experienced by 96% of pts, with neutropenia (82%), febrile neutropenia (49%), and thrombocytopenia (42%) being the most common. Neutropenia (49%), including febrile neutropenia (45%), was the most common serious TEAE. The 30-day mortality rate after first dose was 1%. The intention-to-treat ORR was 80%, including complete remission (CR) in 40% and marrow CR (mCR) in 40% (42% of pts with mCR also had hematologic improvement; no partial remissions were recorded. For 31 pts with CR, median time to CR was 2.6 mos (range 1.2–19.6) and median duration of response was 13.8 mos (95% CI 8.9,–). Post-baseline transfusion independence rate (defined as no transfusion ≥8 weeks), for pts who were transfusion-dependent for RBC and/or platelets at baseline, was 46.5%. Median OS of the study population was 28.2 mos (95% CI 17.7, –). Median OS for 31 pts achieving CR was 28.6 mos (95% CI 27.5, –). 23% of the study population moved on to post-study allogeneic HSCT (incl. BM and peripheral blood stem cell).
Conclusion
The combination of Ven+Aza was associated with a rapid and durable response, promising efficacy including remission rates and OS, and an acceptable safety profile for pts with HR-MDS. Additional correlation with disease risk features, including TP53 mutations, will be presented.
Keyword(s): Azacitidine, BCL2, MDS
Abstract: EP917
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
Hypomethylating agents (HMA) are the current standard of care for patients (pts) with higher-risk myelodysplastic syndromes (HR-MDS) who are ineligible for allogeneic hematopoietic stem cell transplantation (HSCT). However, overall response rates (ORRs) remain suboptimal in pts receiving azacitidine (Aza), and their expected median overall survival (OS) is less than 2 years. Venetoclax (Ven) is a selective, potent, oral BCL-2 inhibitor, which has demonstrated synergy with HMAs in preclinical and clinical studies of myeloid malignancies.
Aims
We report updated safety and efficacy in pts treated in an ongoing phase 1b study (NCT02942290) evaluating Ven+Aza for treatment-naïve HR-MDS.
Methods
Pts (≥18 years) with International Prognostic Scoring System (IPSS) intermediate-2 or high-risk MDS, bone marrow (BM) blasts <20% at baseline and ECOG ≤2 performance status were enrolled. Aza 75 mg/m2 was administered on Days (d) 1–7 of each 28-d cycle. Ven was initially given at a dose of 400 mg or 800 mg daily in 28-d cycles. Due to poor tolerance among pts with MDS, the study was amended to examine feasibility of several dose cohorts (100, 200 and 400 mg x 14 d each 28d cycle). Primary objectives were to assess the Ven+Aza safety profile and to establish the RP2D. Key secondary objectives included assessment of ORR and OS. Safety and efficacy analyses were carried out on all pts who received ≥1 dose of study drug and efficacy endpoints evaluated per IWG 2006 response criteria. Neutropenia included neutropenia, febrile neutropenia, neutrophil count decreased, neutropenic sepsis/infection, agranulocytosis. Thrombocytopenia included thrombocytopenia and platelet count decreased. Baseline mutational status was evaluated on BM aspirate samples using the Archer VariantPlex Myeloid panel.
Results
At data cutoff Dec 15, 2020, 78 pts had received Ven+Aza (51 pts received Ven at the RP2D of 400 mg x 14 d), with a median follow-up time of 23 mos (range 0.1–44.2). Median age was 70 years (range 26–87); 72% male; and 90% had excess BM blasts (>5 to ≤10%, n=21; >10 to ≤20%, n=49). Grade 3/4 neutropenia (59%) or thrombocytopenia (33%) were present at baseline. All pts experienced ≥1 treatment-emergent adverse event (TEAE), the most common being neutropenia (83%), nausea (55%), and constipation (54%). Grade 3/4 TEAEs were experienced by 96% of pts, with neutropenia (82%), febrile neutropenia (49%), and thrombocytopenia (42%) being the most common. Neutropenia (49%), including febrile neutropenia (45%), was the most common serious TEAE. The 30-day mortality rate after first dose was 1%. The intention-to-treat ORR was 80%, including complete remission (CR) in 40% and marrow CR (mCR) in 40% (42% of pts with mCR also had hematologic improvement; no partial remissions were recorded. For 31 pts with CR, median time to CR was 2.6 mos (range 1.2–19.6) and median duration of response was 13.8 mos (95% CI 8.9,–). Post-baseline transfusion independence rate (defined as no transfusion ≥8 weeks), for pts who were transfusion-dependent for RBC and/or platelets at baseline, was 46.5%. Median OS of the study population was 28.2 mos (95% CI 17.7, –). Median OS for 31 pts achieving CR was 28.6 mos (95% CI 27.5, –). 23% of the study population moved on to post-study allogeneic HSCT (incl. BM and peripheral blood stem cell).

Conclusion
The combination of Ven+Aza was associated with a rapid and durable response, promising efficacy including remission rates and OS, and an acceptable safety profile for pts with HR-MDS. Additional correlation with disease risk features, including TP53 mutations, will be presented.
Keyword(s): Azacitidine, BCL2, MDS


