
Contributions
Abstract: EP916
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
Real-world studies have shown that persistence with intravenous (IV) and subcutaneous (SC) hypomethylating agents (HMAs) among patients with higher-risk myelodysplastic syndromes (MDS) is poor, with over one-third of treated patients receiving <4 cycles or having a ≥90-day gap in therapy, despite recommendations for at least 4-6 cycles to elicit response in the absence of progression/unacceptable toxicity. Survival outcomes have also been shown to be worse, and direct medical costs higher, among HMA non-persistent versus persistent patients.
Aims
To explore factors associated with early discontinuation of HMA therapy among patients with higher-risk MDS in the real-world setting.
Methods
This was a retrospective cohort study among patients from the 2010-2016 SEER-Medicare linked database with a diagnosis of refractory anaemia with excess blasts (RAEB; a surrogate for higher-risk MDS) from 2011-2015. Included patients had to have received HMA therapy and have ≥12 months’ continuous follow-up after diagnosis. Discontinuation was defined as stopping HMA therapy before 4 cycles. Multivariable logistic regression was used to assess predictors of HMA discontinuation.
Results
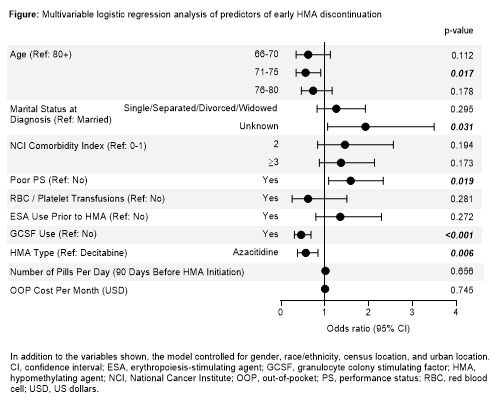
In total, 664 patients with RAEB and treated with HMAs were included. Overall, 193 patients (29%) discontinued before receiving 4 cycles of therapy; of these, 91 (47%) discontinued after 1 cycle, 57 (30%) after 2 cycles, and 45 (23%) after 3 cycles. Compared with patients continuing for ≥4 cycles, patients discontinuing before 4 cycles were generally older (mean age 79.1 vs 77.3 years; p<0.001) and more likely to be single/separated/divorced/widowed (35% vs 30%; p=0.024), have more comorbidities (National Cancer Institute [NCI] Comorbidity Index ≥3, 36% vs 26%; p=0.004), and have poor performance status (PS) (55% vs 40%; p<0.001). These trends were most pronounced among patients discontinuing HMA therapy after only 1 cycle vs ≥4 cycles (mean age, 79.2 vs 77.3 years; single/separated/divorced/widowed, 39% vs 30%; NCI Comorbidity Index ≥3, 41% vs 26%; poor PS, 63% vs 40%; p<0.05 for all comparisons). In multivariable analysis, age 71-75 vs ≥80 years (odds ratio [OR] 0.556, p=0.017) and poor PS (OR 1.585, p=0.019) remained significant predictors of HMA discontinuation. Among treatment-related factors, the most statistically significant association with HMA discontinuation was observed for granulocyte colony-stimulating factor (GCSF) use (OR 0.453, p<0.001). Number of pills/day in the 90 days before HMA initiation was not a predictor of HMA discontinuation (OR 1.009, p=0.656) (Figure).
Conclusion
In this real-world study, almost one-third of RAEB patients treated with IV/SC HMAs discontinued before 4 cycles, with almost half of these patients discontinuing after only 1 cycle. Predictors of HMA discontinuation included older age and poor PS. Novel therapeutic approaches and interventions are needed to improve persistence with HMA therapy, particularly among these higher-risk groups. Further research is also warranted to fully elucidate the reasons for early discontinuation in this population.
Keyword(s): Myelodysplasia
Abstract: EP916
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
Real-world studies have shown that persistence with intravenous (IV) and subcutaneous (SC) hypomethylating agents (HMAs) among patients with higher-risk myelodysplastic syndromes (MDS) is poor, with over one-third of treated patients receiving <4 cycles or having a ≥90-day gap in therapy, despite recommendations for at least 4-6 cycles to elicit response in the absence of progression/unacceptable toxicity. Survival outcomes have also been shown to be worse, and direct medical costs higher, among HMA non-persistent versus persistent patients.
Aims
To explore factors associated with early discontinuation of HMA therapy among patients with higher-risk MDS in the real-world setting.
Methods
This was a retrospective cohort study among patients from the 2010-2016 SEER-Medicare linked database with a diagnosis of refractory anaemia with excess blasts (RAEB; a surrogate for higher-risk MDS) from 2011-2015. Included patients had to have received HMA therapy and have ≥12 months’ continuous follow-up after diagnosis. Discontinuation was defined as stopping HMA therapy before 4 cycles. Multivariable logistic regression was used to assess predictors of HMA discontinuation.
Results
In total, 664 patients with RAEB and treated with HMAs were included. Overall, 193 patients (29%) discontinued before receiving 4 cycles of therapy; of these, 91 (47%) discontinued after 1 cycle, 57 (30%) after 2 cycles, and 45 (23%) after 3 cycles. Compared with patients continuing for ≥4 cycles, patients discontinuing before 4 cycles were generally older (mean age 79.1 vs 77.3 years; p<0.001) and more likely to be single/separated/divorced/widowed (35% vs 30%; p=0.024), have more comorbidities (National Cancer Institute [NCI] Comorbidity Index ≥3, 36% vs 26%; p=0.004), and have poor performance status (PS) (55% vs 40%; p<0.001). These trends were most pronounced among patients discontinuing HMA therapy after only 1 cycle vs ≥4 cycles (mean age, 79.2 vs 77.3 years; single/separated/divorced/widowed, 39% vs 30%; NCI Comorbidity Index ≥3, 41% vs 26%; poor PS, 63% vs 40%; p<0.05 for all comparisons). In multivariable analysis, age 71-75 vs ≥80 years (odds ratio [OR] 0.556, p=0.017) and poor PS (OR 1.585, p=0.019) remained significant predictors of HMA discontinuation. Among treatment-related factors, the most statistically significant association with HMA discontinuation was observed for granulocyte colony-stimulating factor (GCSF) use (OR 0.453, p<0.001). Number of pills/day in the 90 days before HMA initiation was not a predictor of HMA discontinuation (OR 1.009, p=0.656) (Figure).

Conclusion
In this real-world study, almost one-third of RAEB patients treated with IV/SC HMAs discontinued before 4 cycles, with almost half of these patients discontinuing after only 1 cycle. Predictors of HMA discontinuation included older age and poor PS. Novel therapeutic approaches and interventions are needed to improve persistence with HMA therapy, particularly among these higher-risk groups. Further research is also warranted to fully elucidate the reasons for early discontinuation in this population.
Keyword(s): Myelodysplasia


