
Contributions
Abstract: EP913
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
Median overall survival after failure of hypomethylating agents (HMAs) for patients with myelodysplastic syndrome (MDS) is 5-6 months. Preclinical and clinical data suggest that myeloblasts express PDL-1 and that HMAs induce T-cell PD-1 expression, which may promote resistance or relapse despite effective demethylation.
Aims
To determine the safety profile, efficacy and impact on overall survival of atezolizumab (atezo), a PDL-1 inhibitor, in combination with the decitabine and deoxyguanosine dinucleotide guadecitabine (guaD), for patients with MDS and CMML treated after failure of an approved HMA.
Methods
We conducted a Phase I/II clinical trial for adult patients with relapsed or refractory, intermediate (3+) or high-risk MDS by the revised international scoring system (IPSS-R), or CMML, after failure of a standard HMA. The primary endpoint of Phase I was to determine a safe dose of guaD in combination with a fixed dose of atezo. A 3x3 dose escalation design for guaD was used, beginning with 30mg/m2 (Dose level -1) SC days 1-5 with atezo 840mg IV days 8 and 22 of a 28-day cycle. Escalation to the recommended dose of guaD, 60 mg/m2 (Dose level 1), was allowed if no dose-limiting toxicities (DLTs) were observed at Dose level -1. If no DLTs were observed at Dose level 1, 3 additional patients were treated to confirm safety. Phase II was planned to include 30 patients treated at Dose level 1, with the primary endpoint of efficacy measured by overall response rate (ORR), based on the 2006 IWG Response Criteria for MDS. Overall survival (OS) was a secondary endpoint.
Results
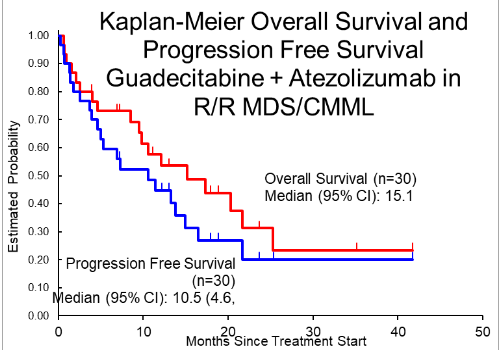
Between June 2017 and October 2020, 33 patients from 4 centers enrolled in the study. Median age was 73 (54-85) years, 22 (67%) were male, 11 (33%) had IPSS-R intermediate-risk and 20 (64%) had high- or very high risk MDS, 2 had CMML, and two-thirds were refractory to their prior HMA. No DLTs occurred in 3 patients treated at Dose level -1. No DLTs were seen among 6 patients treated at Dose level 1, so 24 additional patients were treated. The most common grade 4 toxicities at Dose level 1, regardless of attribution, were thrombocytopenia in 11 (36.7%), neutropenia in 10 (33.3%), and anemia in 5 (16.7%) patients. There were 4 grade 3 events considered autoimmune and attributed to atezolizumab – 2 encephalitis and 2 pneumonitis. Four deaths occurred while on therapy, including 1 patient with sepsis and brainstem herniation after lumbar puncture; 11 patients came off treatment due to disease progression, 3 due to clinical deterioration, 2 at their own request, 4 at physician’s discretion, and 5 patients due to unacceptable toxicity. A median of 5 cycles (1-21) were administered and median follow-up is 25.3 (3.9-41.7) months with 4 patients still on treatment. ORR is 30% (CI 16-41%), with 2 CR (6%), 4 marrow CR (12%), 1 PR and 3 (9%) Hematologic Improvement. Median overall survival is 15.1 (8.5, 21.6) months among the 30 patients treated at Dose level 1, 17.3 (8.5, 27.3) months among those refractory to prior HMA and 10.5 (4.4, 21.6) months in patients who responded and then relapsed after prior HMA (p=0.58). There was a trend for better survival among females, 21.6 (10.5, 41.7+) vs 12.1 (2.5, 20.3) months (p=0.068).
Conclusion
In this phase I/II trial of patients with higher risk, predominantly HMA-refractory MDS, guadecitabine with atezolizumab yielded modest IWG responses, but overall survival appears better than predicted for this patient population, particularly among women. Ongoing correlative work will investigate immunologic characteristics of long-term survivors.
Keyword(s): Epigenetic, Immune therapy, MDS
Abstract: EP913
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
Median overall survival after failure of hypomethylating agents (HMAs) for patients with myelodysplastic syndrome (MDS) is 5-6 months. Preclinical and clinical data suggest that myeloblasts express PDL-1 and that HMAs induce T-cell PD-1 expression, which may promote resistance or relapse despite effective demethylation.
Aims
To determine the safety profile, efficacy and impact on overall survival of atezolizumab (atezo), a PDL-1 inhibitor, in combination with the decitabine and deoxyguanosine dinucleotide guadecitabine (guaD), for patients with MDS and CMML treated after failure of an approved HMA.
Methods
We conducted a Phase I/II clinical trial for adult patients with relapsed or refractory, intermediate (3+) or high-risk MDS by the revised international scoring system (IPSS-R), or CMML, after failure of a standard HMA. The primary endpoint of Phase I was to determine a safe dose of guaD in combination with a fixed dose of atezo. A 3x3 dose escalation design for guaD was used, beginning with 30mg/m2 (Dose level -1) SC days 1-5 with atezo 840mg IV days 8 and 22 of a 28-day cycle. Escalation to the recommended dose of guaD, 60 mg/m2 (Dose level 1), was allowed if no dose-limiting toxicities (DLTs) were observed at Dose level -1. If no DLTs were observed at Dose level 1, 3 additional patients were treated to confirm safety. Phase II was planned to include 30 patients treated at Dose level 1, with the primary endpoint of efficacy measured by overall response rate (ORR), based on the 2006 IWG Response Criteria for MDS. Overall survival (OS) was a secondary endpoint.
Results
Between June 2017 and October 2020, 33 patients from 4 centers enrolled in the study. Median age was 73 (54-85) years, 22 (67%) were male, 11 (33%) had IPSS-R intermediate-risk and 20 (64%) had high- or very high risk MDS, 2 had CMML, and two-thirds were refractory to their prior HMA. No DLTs occurred in 3 patients treated at Dose level -1. No DLTs were seen among 6 patients treated at Dose level 1, so 24 additional patients were treated. The most common grade 4 toxicities at Dose level 1, regardless of attribution, were thrombocytopenia in 11 (36.7%), neutropenia in 10 (33.3%), and anemia in 5 (16.7%) patients. There were 4 grade 3 events considered autoimmune and attributed to atezolizumab – 2 encephalitis and 2 pneumonitis. Four deaths occurred while on therapy, including 1 patient with sepsis and brainstem herniation after lumbar puncture; 11 patients came off treatment due to disease progression, 3 due to clinical deterioration, 2 at their own request, 4 at physician’s discretion, and 5 patients due to unacceptable toxicity. A median of 5 cycles (1-21) were administered and median follow-up is 25.3 (3.9-41.7) months with 4 patients still on treatment. ORR is 30% (CI 16-41%), with 2 CR (6%), 4 marrow CR (12%), 1 PR and 3 (9%) Hematologic Improvement. Median overall survival is 15.1 (8.5, 21.6) months among the 30 patients treated at Dose level 1, 17.3 (8.5, 27.3) months among those refractory to prior HMA and 10.5 (4.4, 21.6) months in patients who responded and then relapsed after prior HMA (p=0.58). There was a trend for better survival among females, 21.6 (10.5, 41.7+) vs 12.1 (2.5, 20.3) months (p=0.068).

Conclusion
In this phase I/II trial of patients with higher risk, predominantly HMA-refractory MDS, guadecitabine with atezolizumab yielded modest IWG responses, but overall survival appears better than predicted for this patient population, particularly among women. Ongoing correlative work will investigate immunologic characteristics of long-term survivors.
Keyword(s): Epigenetic, Immune therapy, MDS


