
Contributions
Abstract: EP910
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
IMerge (MDS3001, NCT02598661) is a Phase 2/3 global study of imetelstat, a first-in-class telomerase inhibitor, for red blood cell (RBC) transfusion dependent (TD) non-del(5q) patients (pts) with ESA-R/R LR-MDS. Phase 2 results indicated that imetelstat achieved durable transfusion independence (TI) with a manageable safety profile (Steensma ASH 2018; Fenaux EHA 2019; Steensma JCO 2020). With a median follow-up of 24 months for Phase 2, 42%, 32% and 29% of 38 pts achieved ≥8-week (w), ≥24-w and 1-year (y) TI, respectively (Platzbecker EHA 2020).
Aims
To evaluate clinical efficacy of imetelstat in molecularly defined subtypes based on cytogenetic and mutation profiles.
Methods
At screening, bone marrow aspirates were used for cytogenetic analysis by karyotyping. Peripheral blood samples were collected to analyze mutations by next-generation sequencing using the Illumina TruSight Myeloid Panel of 54 genes. Correlation analyses between molecular profiles and clinical efficacy, including TI ≥8-w, ≥24-w, ≥1-y, and hematologic improvement-erythroid (HI-E) response per International Working Group 2006 guidelines, were performed for pts in the Phase 2 part of IMerge study.
Results
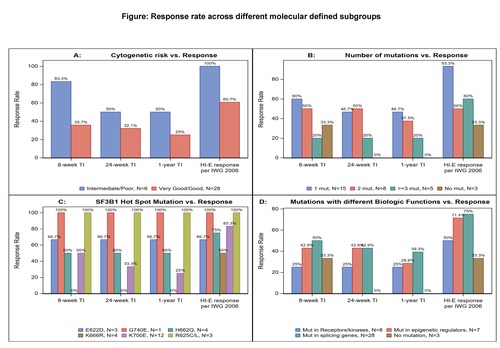
Of 34/38 pts with baseline cytogenetic results, 28 (82.4%) pts had very good/good and 6 (17.6%) pts had intermediate (int)/poor cytogenetic risk (n=3 trisomy 8, n=1 for del(7q), inv(3)/t(3q) and complex karyotype). Though pts with int/poor risk had higher TI and HI-E response rates compared with pts with very good/good risk pts, no statistically significant difference was observed: 83.3% (5/6) vs 35.7% (10/28) had ≥8-w TI; 50% (3/6) vs 32.1% (9/28) had ≥24-w TI; and 50% (3/6) vs 25% (7/28) had ≥1-y TI; 100% (6/6) of pts with int/poor risk group vs 60.7% (17/28) achieved HI-E response (Fig. A).
Of 31/38 pts with baseline mutation data, 28 (90.3%) pts had at least one mutation, among which 15 (53.6%), 8 (28.6%) and 5 (17.9%) pts had 1, 2 and ≥3 mutations, respectively. The ≥8-w TI rate was 60%, 50% and 20% respectively, ≥1-y TI rate was 46.7%, 37.5% and 20% respectively, and HI-E response rate was 93.3%, 50% and 60%, respectively (Fig. B). Three pts (9.7%) had no mutation detected, of which 1 pt had 8-w TI and HI-E response. The most frequently mutated gene was SF3B1 (87.1%, n=27), consistent with the predominance of ring sideroblast phenotypes (n=23); followed by DNMT3A (19.4%), KIT (16.1%) and RUNX1 (6.4%), all coexisting with SF3B1 mutation. Among pts with SF3B1 mutation, hot spot mutations were detected: 3 (11.1%) E622D, 3 (11.1%) R625C/L, 4 (14.8%) H662Q, 4 (14.8%) K666R, 12 (44.4%) K700E and 1 (3.7%%) G740E, respectively. Durable TI was observed in pts with these hot spot mutations except K666R, and HI-E response was seen in pts with all hot spot mutations (Fig. C). Analysis of clinical responses across the mutation defined subgroups on genes involved in different biological functions, including splicing process, epigenetic regulation, or receptors/kinases, were performed and ≥8-w TI was 50.0%, 42.9% and 25.0%; ≥24-w TI was 42.9%, 42.9% and 25.0%; ≥1-y TI was 39.3%, 28.6% and 25.0%, and HI-E responses were 75.0%, 71.4% and 50.0%, respectively (Fig. D).
Conclusion
Imetelstat demonstrated clinical efficacy across different molecularly defined subgroups including in pts with poor prognosis in heavily transfused LR-MDS ESA-R/R, who have limited treatment options. Phase 3 of IMerge, a placebo-controlled trial of imetelstat, is ongoing to further confirm the efficacy, safety, and biomarker results.
Keyword(s): Cytogenetics, MDS, Myelodysplasia, Telomerase
Abstract: EP910
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Clinical
Background
IMerge (MDS3001, NCT02598661) is a Phase 2/3 global study of imetelstat, a first-in-class telomerase inhibitor, for red blood cell (RBC) transfusion dependent (TD) non-del(5q) patients (pts) with ESA-R/R LR-MDS. Phase 2 results indicated that imetelstat achieved durable transfusion independence (TI) with a manageable safety profile (Steensma ASH 2018; Fenaux EHA 2019; Steensma JCO 2020). With a median follow-up of 24 months for Phase 2, 42%, 32% and 29% of 38 pts achieved ≥8-week (w), ≥24-w and 1-year (y) TI, respectively (Platzbecker EHA 2020).
Aims
To evaluate clinical efficacy of imetelstat in molecularly defined subtypes based on cytogenetic and mutation profiles.
Methods
At screening, bone marrow aspirates were used for cytogenetic analysis by karyotyping. Peripheral blood samples were collected to analyze mutations by next-generation sequencing using the Illumina TruSight Myeloid Panel of 54 genes. Correlation analyses between molecular profiles and clinical efficacy, including TI ≥8-w, ≥24-w, ≥1-y, and hematologic improvement-erythroid (HI-E) response per International Working Group 2006 guidelines, were performed for pts in the Phase 2 part of IMerge study.
Results
Of 34/38 pts with baseline cytogenetic results, 28 (82.4%) pts had very good/good and 6 (17.6%) pts had intermediate (int)/poor cytogenetic risk (n=3 trisomy 8, n=1 for del(7q), inv(3)/t(3q) and complex karyotype). Though pts with int/poor risk had higher TI and HI-E response rates compared with pts with very good/good risk pts, no statistically significant difference was observed: 83.3% (5/6) vs 35.7% (10/28) had ≥8-w TI; 50% (3/6) vs 32.1% (9/28) had ≥24-w TI; and 50% (3/6) vs 25% (7/28) had ≥1-y TI; 100% (6/6) of pts with int/poor risk group vs 60.7% (17/28) achieved HI-E response (Fig. A).
Of 31/38 pts with baseline mutation data, 28 (90.3%) pts had at least one mutation, among which 15 (53.6%), 8 (28.6%) and 5 (17.9%) pts had 1, 2 and ≥3 mutations, respectively. The ≥8-w TI rate was 60%, 50% and 20% respectively, ≥1-y TI rate was 46.7%, 37.5% and 20% respectively, and HI-E response rate was 93.3%, 50% and 60%, respectively (Fig. B). Three pts (9.7%) had no mutation detected, of which 1 pt had 8-w TI and HI-E response. The most frequently mutated gene was SF3B1 (87.1%, n=27), consistent with the predominance of ring sideroblast phenotypes (n=23); followed by DNMT3A (19.4%), KIT (16.1%) and RUNX1 (6.4%), all coexisting with SF3B1 mutation. Among pts with SF3B1 mutation, hot spot mutations were detected: 3 (11.1%) E622D, 3 (11.1%) R625C/L, 4 (14.8%) H662Q, 4 (14.8%) K666R, 12 (44.4%) K700E and 1 (3.7%%) G740E, respectively. Durable TI was observed in pts with these hot spot mutations except K666R, and HI-E response was seen in pts with all hot spot mutations (Fig. C). Analysis of clinical responses across the mutation defined subgroups on genes involved in different biological functions, including splicing process, epigenetic regulation, or receptors/kinases, were performed and ≥8-w TI was 50.0%, 42.9% and 25.0%; ≥24-w TI was 42.9%, 42.9% and 25.0%; ≥1-y TI was 39.3%, 28.6% and 25.0%, and HI-E responses were 75.0%, 71.4% and 50.0%, respectively (Fig. D).

Conclusion
Imetelstat demonstrated clinical efficacy across different molecularly defined subgroups including in pts with poor prognosis in heavily transfused LR-MDS ESA-R/R, who have limited treatment options. Phase 3 of IMerge, a placebo-controlled trial of imetelstat, is ongoing to further confirm the efficacy, safety, and biomarker results.
Keyword(s): Cytogenetics, MDS, Myelodysplasia, Telomerase


