
Contributions
Abstract: EP896
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Biology & Translational Research
Background
Clonal hematopoiesis (CH) is a common age-related condition predisposing to blood cancer and cardiovascular disease (CVD). Murine models demonstrate CH-mediated altered immune function and proinflammation. Low-grade inflammation has been implicated in the pathogenesis of osteoarthritis (OA), the main indication for total hip arthroplasty (THA). THA-derived hip bones serve as a major source of ‘healthy’ hematopoietic cells in experimental hematology.
Aims
We prospectively investigated frequency and clinical associations of CH in 200 patients without known hematologic disease undergoing THA for OA.
Methods
BM samples were collected from 200 patients without known hematologic disease undergoing THA for OA between 07/2017 and 08/2020 after written informed consent. The study was approved by the respective ethics committees in accordance with the Declaration of Helsinki. Targeted sequencing of 68 genes recurrently mutated in hematologic malignancies identified variants with a VAF threshold of ≥1%. For correlative analyses of clinical parameters, only variants fulfilling the current CHIP definition (clonal hematopoiesis of indeterminate potential, defined as somatic variants with allele frequencies [VAF] ≥2%) were included. Statistical analysis was performed with GraphPad Prism v6.0 and R v3.6.3.
Results
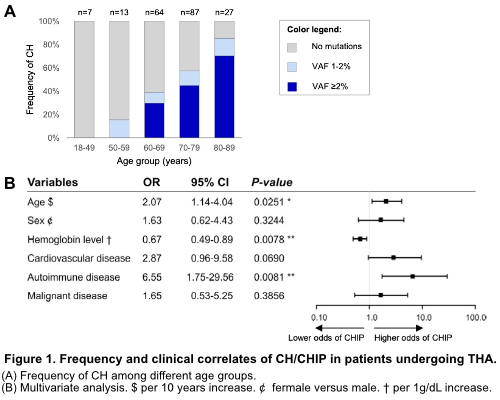
Prevalence of CH was 50.0%, including 77 patients with CHIP, and 23 patients harboring CH with lower mutation burden (VAF 1-2%). CH became progressively more frequent with age (Figure 1A). Most commonly mutated genes were DNMT3A (29.5%), TET2 (15.0%) and ASXL1 (3.5%). Most patients (n=56) had 1 mutation, 32 harbored 2 variants, and 10 carried 3 mutations, while two patients harbored 4 or 5 mutations, respectively. Number of variants per individual correlated with age (p=0.031), whereas VAF (median 2.7%, range 1.0-32.7%) did not (p=0.73). CHIP significantly correlated with older age (median, 74.0 years [y] with CHIP vs. 68.5 y without, respectively; p<0.00010), lower hemoglobin levels (median, 12.7 g/dL vs. 13.7 g/dL; p=0.0020) and higher MCV (median, 91.8 fl vs. 89.0 fl; p=0.0076). Of note, 66.7% of the patients with anemia had detectable mutations with VAF≥2% (who would thus be classified as clonal cytopenia of uncertain significance [CCUS]), whereas prevalence of CHIP was lower in patients with normal hemoglobin levels (32.7%, p=0.0014). Furthermore, we observed an enrichment of SF3B1 and TP53 mutations in anemic patients compared to those with normal blood counts (p=0.020). CHIP carriers were more likely to have CVD (p=0.0080), as previously reported, and present/prior malignancy (p=0.029). Strikingly, CHIP also associated with presence of autoimmune disease (AID, p=0.034), comprising diverse autoimmune disorders. Multivariate analysis with adjustment for age and sex confirmed significant associations between CHIP and older age (p=0.025), lower hemoglobin levels (p=0.0078), and AID (p=0.0081), but not with CVD or malignancy (Figure 1B).
Conclusion
Prevalence of CH in our prospectively enrolled cohort of older adults undergoing THA for OA is considerably higher than previously reported in healthy individuals or specific patient groups, such as those with ischemic heart failure or CVD. Together, these findings underscore the association between CH and inflammatory diseases. Our results have considerable relevance for management of patients with OA, AID and anemia, and question the use of hip bone-derived cells as ‘healthy’ experimental controls.
Keyword(s): Autoimmune disease, Clonality, Hematopoiesis, Hematopoietic cell
Abstract: EP896
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Biology & Translational Research
Background
Clonal hematopoiesis (CH) is a common age-related condition predisposing to blood cancer and cardiovascular disease (CVD). Murine models demonstrate CH-mediated altered immune function and proinflammation. Low-grade inflammation has been implicated in the pathogenesis of osteoarthritis (OA), the main indication for total hip arthroplasty (THA). THA-derived hip bones serve as a major source of ‘healthy’ hematopoietic cells in experimental hematology.
Aims
We prospectively investigated frequency and clinical associations of CH in 200 patients without known hematologic disease undergoing THA for OA.
Methods
BM samples were collected from 200 patients without known hematologic disease undergoing THA for OA between 07/2017 and 08/2020 after written informed consent. The study was approved by the respective ethics committees in accordance with the Declaration of Helsinki. Targeted sequencing of 68 genes recurrently mutated in hematologic malignancies identified variants with a VAF threshold of ≥1%. For correlative analyses of clinical parameters, only variants fulfilling the current CHIP definition (clonal hematopoiesis of indeterminate potential, defined as somatic variants with allele frequencies [VAF] ≥2%) were included. Statistical analysis was performed with GraphPad Prism v6.0 and R v3.6.3.
Results
Prevalence of CH was 50.0%, including 77 patients with CHIP, and 23 patients harboring CH with lower mutation burden (VAF 1-2%). CH became progressively more frequent with age (Figure 1A). Most commonly mutated genes were DNMT3A (29.5%), TET2 (15.0%) and ASXL1 (3.5%). Most patients (n=56) had 1 mutation, 32 harbored 2 variants, and 10 carried 3 mutations, while two patients harbored 4 or 5 mutations, respectively. Number of variants per individual correlated with age (p=0.031), whereas VAF (median 2.7%, range 1.0-32.7%) did not (p=0.73). CHIP significantly correlated with older age (median, 74.0 years [y] with CHIP vs. 68.5 y without, respectively; p<0.00010), lower hemoglobin levels (median, 12.7 g/dL vs. 13.7 g/dL; p=0.0020) and higher MCV (median, 91.8 fl vs. 89.0 fl; p=0.0076). Of note, 66.7% of the patients with anemia had detectable mutations with VAF≥2% (who would thus be classified as clonal cytopenia of uncertain significance [CCUS]), whereas prevalence of CHIP was lower in patients with normal hemoglobin levels (32.7%, p=0.0014). Furthermore, we observed an enrichment of SF3B1 and TP53 mutations in anemic patients compared to those with normal blood counts (p=0.020). CHIP carriers were more likely to have CVD (p=0.0080), as previously reported, and present/prior malignancy (p=0.029). Strikingly, CHIP also associated with presence of autoimmune disease (AID, p=0.034), comprising diverse autoimmune disorders. Multivariate analysis with adjustment for age and sex confirmed significant associations between CHIP and older age (p=0.025), lower hemoglobin levels (p=0.0078), and AID (p=0.0081), but not with CVD or malignancy (Figure 1B).

Conclusion
Prevalence of CH in our prospectively enrolled cohort of older adults undergoing THA for OA is considerably higher than previously reported in healthy individuals or specific patient groups, such as those with ischemic heart failure or CVD. Together, these findings underscore the association between CH and inflammatory diseases. Our results have considerable relevance for management of patients with OA, AID and anemia, and question the use of hip bone-derived cells as ‘healthy’ experimental controls.
Keyword(s): Autoimmune disease, Clonality, Hematopoiesis, Hematopoietic cell


