
Contributions
Abstract: EP892
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Biology & Translational Research
Background
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal stem cell neoplasms associated with cytopenias. Diagnosis is based on peripheral blood count, bone marrow morphology and cytogenetics. In addition to cytogenetics, which allows risk stratification of patients, multiple recurrent mutations have been identified in recent years. For some diseases, a gender-dependent prevalence of mutations and their impact on outcome have been described, but a systematic evaluation of the mutational landscape and its impact on the prognosis of patients in relation to their gender in MDS is still lacking.
Aims
To compare the molecular genetic landscape and identify potential different outcomes between female and male patients with MDS.
Methods
The cohort comprised 588 patients (234 female and 354 male) with a median age of 72, diagnosed as MDS by cytomorphology and cytogenetics following WHO classification. All patients were previously investigated by next generation deep sequencing (Haferlach et al, Leukemia 2014) and WGS. All data were analyzed for 4 virtual gene panels, the 12 genes as given in the MDS NCCN guidelines with a incidence >5% (Version 2.2020), the 34 gene panel summarized in McClure et al. (J Mol Diag 2018), the 723 cosmic cancer gene census (CCGC, v91) panel and finally the exome.
Results
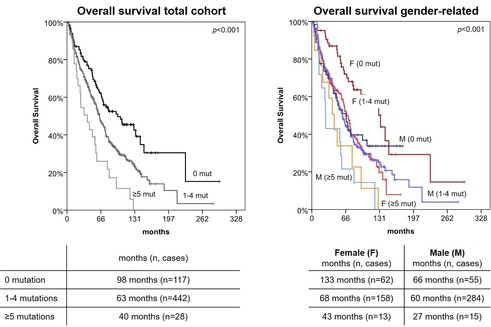
A gender-specific analysis of the MDS subtypes confirmed the already known female predominance in MDS with isolated del(5q) (73% vs 27%; p<0.001), while all other MDS subtypes showed no gender differences. Additionally, female patients showed higher platelet counts than their male counterparts (mean 227,229/µl vs. 180,950/µl; p=0.001). There were no gender-related differences in age, karyotype or IPSS-R score. However, in non del(5q) subtypes female showed predominantly a normal karyotype (79% vs. 67%; p=0.002). Addressing individual gene mutations showed that female patients were more often mutated in DNMT3A (40/234, 17% vs. 24/354, 7%; p<0.001), while males were more often mutated in TET2 (119/354, 34% vs. 58/234, 25%; p=0.027). Interestingly, ZRSR2 mutations (on chr. X) occurred nearly exclusively in male patients (39/354, 11% in male vs. 1/234, 0% in female; p<0.001). Overall the median number of mutations was slightly higher in male patients and the absence of mutations in the myeloid specific gene panels was more pronounced in female patients (NCCN gene panel: 63/234, 27% vs. 55/354, 16%; p=0.001; McClure gene panel: 52/234, 22% vs. 44/354, 12%; p=0.002). The differences in mutation detection rates disappeared with the larger CCGC panel and the exome. In the following, we aimed to investigate whether the higher number of mutations in males also had an impact on patient outcome. While age and karyotype showed prognostic impact in both groups, gender per se did not affect the outcome. However, looking at gender-dependent survival, a prognostic impact becomes apparent by subdividing the myeloid gene panels into groups with no, 1-4, and ≥5 mutations per patient. Surprisingly, men showed no difference in survival between the two groups with no and less than 5 mutations, whereas this was statistically significant in women (figure, NCCN gene panel). This difference decreases with increasing panel size and number of mutations.
Conclusion
Between female and male MDS patients differences in prevalence and mutational landscape occur. The number of mutations within a small panel of 12 recurrently mutated genes show prognostic impact that is predominant in female patients and should therefore be considered for risk stratification.
Keyword(s): Gender, Molecular markers, Mutation status, Prognosis
Abstract: EP892
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Biology & Translational Research
Background
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal stem cell neoplasms associated with cytopenias. Diagnosis is based on peripheral blood count, bone marrow morphology and cytogenetics. In addition to cytogenetics, which allows risk stratification of patients, multiple recurrent mutations have been identified in recent years. For some diseases, a gender-dependent prevalence of mutations and their impact on outcome have been described, but a systematic evaluation of the mutational landscape and its impact on the prognosis of patients in relation to their gender in MDS is still lacking.
Aims
To compare the molecular genetic landscape and identify potential different outcomes between female and male patients with MDS.
Methods
The cohort comprised 588 patients (234 female and 354 male) with a median age of 72, diagnosed as MDS by cytomorphology and cytogenetics following WHO classification. All patients were previously investigated by next generation deep sequencing (Haferlach et al, Leukemia 2014) and WGS. All data were analyzed for 4 virtual gene panels, the 12 genes as given in the MDS NCCN guidelines with a incidence >5% (Version 2.2020), the 34 gene panel summarized in McClure et al. (J Mol Diag 2018), the 723 cosmic cancer gene census (CCGC, v91) panel and finally the exome.
Results
A gender-specific analysis of the MDS subtypes confirmed the already known female predominance in MDS with isolated del(5q) (73% vs 27%; p<0.001), while all other MDS subtypes showed no gender differences. Additionally, female patients showed higher platelet counts than their male counterparts (mean 227,229/µl vs. 180,950/µl; p=0.001). There were no gender-related differences in age, karyotype or IPSS-R score. However, in non del(5q) subtypes female showed predominantly a normal karyotype (79% vs. 67%; p=0.002). Addressing individual gene mutations showed that female patients were more often mutated in DNMT3A (40/234, 17% vs. 24/354, 7%; p<0.001), while males were more often mutated in TET2 (119/354, 34% vs. 58/234, 25%; p=0.027). Interestingly, ZRSR2 mutations (on chr. X) occurred nearly exclusively in male patients (39/354, 11% in male vs. 1/234, 0% in female; p<0.001). Overall the median number of mutations was slightly higher in male patients and the absence of mutations in the myeloid specific gene panels was more pronounced in female patients (NCCN gene panel: 63/234, 27% vs. 55/354, 16%; p=0.001; McClure gene panel: 52/234, 22% vs. 44/354, 12%; p=0.002). The differences in mutation detection rates disappeared with the larger CCGC panel and the exome. In the following, we aimed to investigate whether the higher number of mutations in males also had an impact on patient outcome. While age and karyotype showed prognostic impact in both groups, gender per se did not affect the outcome. However, looking at gender-dependent survival, a prognostic impact becomes apparent by subdividing the myeloid gene panels into groups with no, 1-4, and ≥5 mutations per patient. Surprisingly, men showed no difference in survival between the two groups with no and less than 5 mutations, whereas this was statistically significant in women (figure, NCCN gene panel). This difference decreases with increasing panel size and number of mutations.

Conclusion
Between female and male MDS patients differences in prevalence and mutational landscape occur. The number of mutations within a small panel of 12 recurrently mutated genes show prognostic impact that is predominant in female patients and should therefore be considered for risk stratification.
Keyword(s): Gender, Molecular markers, Mutation status, Prognosis


