
Contributions
Abstract: EP891
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Biology & Translational Research
Background
Decitabine (DAC) is used as first-line therapy in patients with higher-risk (HR) myelodysplastic syndromes (MDS). However, the clinical outcomes of patients treated with DAC as monotherapy were far from satisfactory with overall response rate (ORR) of 33%>55.4% and overall survival (OS) of 17.7-22 months. Some clinical researches reported that the addition of ATRA to DAC increased response rate and prolonged survival of MDS and elderly acute myeloid leukemia (AML) patients. Therefore, the effect and mechanisms of combination therapy with DAC and ATRA have been studied.
Aims
Conducting the study to investigate the efficacy and mechanisms of DAC combined with ATRA on HR MDS both in vitro and in vivo.
Methods
The combination effects of DAC and ATRA were studied via MDS cell lines and primary cells from newly diagnosed MDS and AML patients. The underlying molecular mechanisms were investigated through MDS cell lines. The MOLM-13-luciferase xenograft murine model was used to verify the enhanced cytotoxic effect of combined drugs.
Results
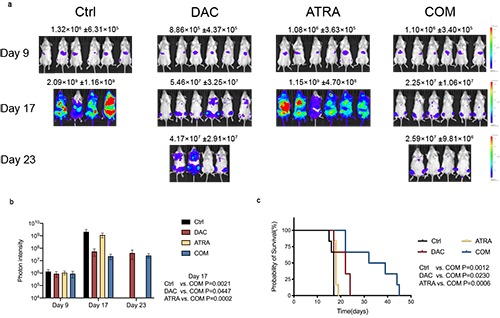
The current study indicated that the strategy combining DAC and ATRA synergistically induced apoptosis of MDS-L and MOLM-13 cells in vitro, and inhibited the viability of primary cells from MDS and AML patients. The combination treatment significantly decreased leukemia burden and prolonged survival of the MOLM-13-luciferase xenograft murine model. Mechanistically, treatment on MDS-L and MOLM-13 cells with DAC as a single agent activated nuclear factor erythroid-2 related factor 2 (Nrf2) signaling pathway. Nrf2 pathway, the master regulator of cellular redox equilibrium, is widely reported to mediate resistance to chemotherapy, demethylation therapy, and target therapy in various tumors. Once activated, Nrf2 leads to the downstream antioxidant response, restrains cellular reactive oxygen species (ROS) accumulation, attenuates ROS-dependent cytotoxicity, thus conferring resistance to DAC. In the current study, the apoptosis of MDS-L and MOLM-13 cells induced by single DAC and combined with ATRA can be reversed by pretreatment with the ROS scavenger N-acetylcysteine (NAC), indicating that the cytotoxicity induced by both monotherapy and combination therapy were ROS dependent. Co-treatment with DAC and ATRA activated the RARα-Nrf2 complex in the nucleus, and reduced binding of HDAC1 to the functional complex, which prevented Nrf2 from being activated by DAC alone, promoted ROS accumulation and enhanced ROS-dependent cytotoxicity, thus overcoming the resistance of MDS cells to DAC.
Conclusion
The interaction between DAC and ARTA exerts collaborative cytotoxicity in MDS cells. Therefore, the regimen combining DAC and ATRA in higher-risk MDS holds potential for clinical application and deserves further explorations.
Keyword(s): Decitabine, MDS/AML, Reactive oxygen species
Abstract: EP891
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Biology & Translational Research
Background
Decitabine (DAC) is used as first-line therapy in patients with higher-risk (HR) myelodysplastic syndromes (MDS). However, the clinical outcomes of patients treated with DAC as monotherapy were far from satisfactory with overall response rate (ORR) of 33%>55.4% and overall survival (OS) of 17.7-22 months. Some clinical researches reported that the addition of ATRA to DAC increased response rate and prolonged survival of MDS and elderly acute myeloid leukemia (AML) patients. Therefore, the effect and mechanisms of combination therapy with DAC and ATRA have been studied.
Aims
Conducting the study to investigate the efficacy and mechanisms of DAC combined with ATRA on HR MDS both in vitro and in vivo.
Methods
The combination effects of DAC and ATRA were studied via MDS cell lines and primary cells from newly diagnosed MDS and AML patients. The underlying molecular mechanisms were investigated through MDS cell lines. The MOLM-13-luciferase xenograft murine model was used to verify the enhanced cytotoxic effect of combined drugs.
Results
The current study indicated that the strategy combining DAC and ATRA synergistically induced apoptosis of MDS-L and MOLM-13 cells in vitro, and inhibited the viability of primary cells from MDS and AML patients. The combination treatment significantly decreased leukemia burden and prolonged survival of the MOLM-13-luciferase xenograft murine model. Mechanistically, treatment on MDS-L and MOLM-13 cells with DAC as a single agent activated nuclear factor erythroid-2 related factor 2 (Nrf2) signaling pathway. Nrf2 pathway, the master regulator of cellular redox equilibrium, is widely reported to mediate resistance to chemotherapy, demethylation therapy, and target therapy in various tumors. Once activated, Nrf2 leads to the downstream antioxidant response, restrains cellular reactive oxygen species (ROS) accumulation, attenuates ROS-dependent cytotoxicity, thus conferring resistance to DAC. In the current study, the apoptosis of MDS-L and MOLM-13 cells induced by single DAC and combined with ATRA can be reversed by pretreatment with the ROS scavenger N-acetylcysteine (NAC), indicating that the cytotoxicity induced by both monotherapy and combination therapy were ROS dependent. Co-treatment with DAC and ATRA activated the RARα-Nrf2 complex in the nucleus, and reduced binding of HDAC1 to the functional complex, which prevented Nrf2 from being activated by DAC alone, promoted ROS accumulation and enhanced ROS-dependent cytotoxicity, thus overcoming the resistance of MDS cells to DAC.

Conclusion
The interaction between DAC and ARTA exerts collaborative cytotoxicity in MDS cells. Therefore, the regimen combining DAC and ATRA in higher-risk MDS holds potential for clinical application and deserves further explorations.
Keyword(s): Decitabine, MDS/AML, Reactive oxygen species


