
Contributions
Abstract: EP889
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Biology & Translational Research
Background
In myelodysplastic syndromes(MDS) in young adults without previous organic dysfunction, the role that germline variants play in its pathogenesis remains to be defined. In fact, the WHO 2017 classification only includes alterations in a single gene, DDX41, in this subgroup of patients. In order to provide knowledge in this group of patients, where these findings could impact allogeneic hematopoietic stem cell transplantation (allo-HSCT) strategies.
Aims
We proposed an exhaustive clinical analysis, favoured by the prospective nature of the study in a Spanish multicentre cohort of patients with data from paired tumor-germinal exome sequencing.
Methods
Prospective cohort study (2014-2020) of 168 patients from 20 Spanish Group of Myelodysplastic Syndrome (GESMD) centers with a de novo diagnosis of MDS between 16-60 years old, without prior organ dysfunction. Collection of 107 clinical variables in relation to family and personal history, diagnosis, treatment, HSCT and disease evolution. Whole exome sequencing(WES) was performed in paired tumor-germline samples with 100x average depth, 150 million reads per sample, and >95% Q30a. WES libraries were prepared using SureSelectXT Target Enrichment System for Illumina Version B.2 and sequenced on a HiSeq4000-NovaSeq6000-Illumina platform. The analysis of the variants was carried out by means of an in-house pipeline: filtering out intronic, synonymous and those variants with MAF>1%. The germline variants were categorized according to ACMG criteria.
Results
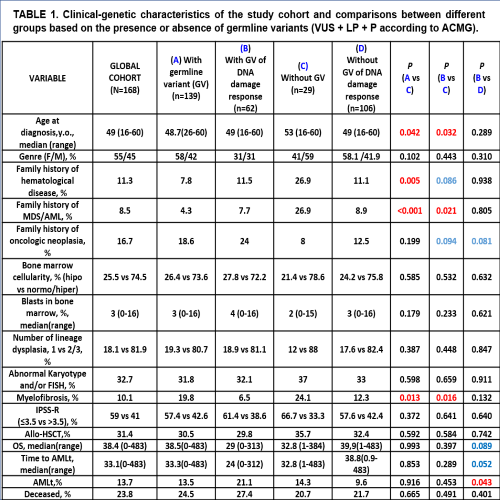
The median age at diagnosis was 49 years old(16-60) with the following WHO 2017 diagnoses: 13.6% MDS-unilineage dysplasia(UD), 11.1% MDS-ring sideroblasts(RS), 32.1% MDS-multilineage dysplasia(MD), 27,1% MDS-excess blasts(EB), 5.6% MDS-isolated deletion-5q(del-5q), 2.5% MDS-unclassifiable(U), 8% chronic myelomonocytic leukemia(CMML). We found germline variants which categorized as pathogenic(P), likely pathogenic(LP) or uncertain significance(VUS) in 82%(n=139) of 168 patients. The frequency of patients with germline variants according to the gene function/pathway were 36%(n=62) DNA damage response, 2%(n=4) telomere function maintenance, 10%(n=18) hematopoiesis regulators, 4.8%(n=8) ribosome function, 11.9%(n=20) immune response,and 32.7%(n=55)others. DDX41(n=3) and SAMD9L(n=1), recurrently implicated in the predisposition to myeloid neoplasia without extra-hemopoietic clinical features, were only present in 4 cases in our cohort. Patients with germline variants(P and/or LP and/or VUS) in DNA repair genes(Table1) showed a lower age(49 vs 53 years old; p=0.032), a higher frequency of family history of MDS/acute myeloid leukemia(AML), and less probability of concomitant myelofibrosis; together with a statistical tendency to present family history of oncologic neoplasia, when compared with those cases without germline alteration. In the overall cohort, the presence of germline variants in DNA repair genes was correlated with superior risk to progression to AML and worse survival. These findings remain statistically significant even when applying a multivariate analysis with IPSS-R dichotomized (≤3.5 vs >3.5).
Conclusion
Young adult patients diagnosed with MDS de novo, without having presented any previous organ dysfunction, show a germline profile enriched in variants affecting genes of the DNA repair pathways. This subgroup presents subtle but relevant clinical differences that would support its specific pathogenicity, adding clinical information to the prediction data in silico.
Keyword(s): Myelodysplasia
Abstract: EP889
Type: E-Poster Presentation
Session title: Myelodysplastic syndromes - Biology & Translational Research
Background
In myelodysplastic syndromes(MDS) in young adults without previous organic dysfunction, the role that germline variants play in its pathogenesis remains to be defined. In fact, the WHO 2017 classification only includes alterations in a single gene, DDX41, in this subgroup of patients. In order to provide knowledge in this group of patients, where these findings could impact allogeneic hematopoietic stem cell transplantation (allo-HSCT) strategies.
Aims
We proposed an exhaustive clinical analysis, favoured by the prospective nature of the study in a Spanish multicentre cohort of patients with data from paired tumor-germinal exome sequencing.
Methods
Prospective cohort study (2014-2020) of 168 patients from 20 Spanish Group of Myelodysplastic Syndrome (GESMD) centers with a de novo diagnosis of MDS between 16-60 years old, without prior organ dysfunction. Collection of 107 clinical variables in relation to family and personal history, diagnosis, treatment, HSCT and disease evolution. Whole exome sequencing(WES) was performed in paired tumor-germline samples with 100x average depth, 150 million reads per sample, and >95% Q30a. WES libraries were prepared using SureSelectXT Target Enrichment System for Illumina Version B.2 and sequenced on a HiSeq4000-NovaSeq6000-Illumina platform. The analysis of the variants was carried out by means of an in-house pipeline: filtering out intronic, synonymous and those variants with MAF>1%. The germline variants were categorized according to ACMG criteria.
Results
The median age at diagnosis was 49 years old(16-60) with the following WHO 2017 diagnoses: 13.6% MDS-unilineage dysplasia(UD), 11.1% MDS-ring sideroblasts(RS), 32.1% MDS-multilineage dysplasia(MD), 27,1% MDS-excess blasts(EB), 5.6% MDS-isolated deletion-5q(del-5q), 2.5% MDS-unclassifiable(U), 8% chronic myelomonocytic leukemia(CMML). We found germline variants which categorized as pathogenic(P), likely pathogenic(LP) or uncertain significance(VUS) in 82%(n=139) of 168 patients. The frequency of patients with germline variants according to the gene function/pathway were 36%(n=62) DNA damage response, 2%(n=4) telomere function maintenance, 10%(n=18) hematopoiesis regulators, 4.8%(n=8) ribosome function, 11.9%(n=20) immune response,and 32.7%(n=55)others. DDX41(n=3) and SAMD9L(n=1), recurrently implicated in the predisposition to myeloid neoplasia without extra-hemopoietic clinical features, were only present in 4 cases in our cohort. Patients with germline variants(P and/or LP and/or VUS) in DNA repair genes(Table1) showed a lower age(49 vs 53 years old; p=0.032), a higher frequency of family history of MDS/acute myeloid leukemia(AML), and less probability of concomitant myelofibrosis; together with a statistical tendency to present family history of oncologic neoplasia, when compared with those cases without germline alteration. In the overall cohort, the presence of germline variants in DNA repair genes was correlated with superior risk to progression to AML and worse survival. These findings remain statistically significant even when applying a multivariate analysis with IPSS-R dichotomized (≤3.5 vs >3.5).

Conclusion
Young adult patients diagnosed with MDS de novo, without having presented any previous organ dysfunction, show a germline profile enriched in variants affecting genes of the DNA repair pathways. This subgroup presents subtle but relevant clinical differences that would support its specific pathogenicity, adding clinical information to the prediction data in silico.
Keyword(s): Myelodysplasia


