Contributions
Abstract: EP885
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
T-cell lymphomas are a pathologically and clinically complex group of heterogenous disorders. Often the interpretation of flow cytometry patterns may present challenges. Identification of an antibody specific for the TCR β constant region 1 (TRBC1) proposes to identify T-cell clonality and distinguish pathological T cells from normal T cells.
Aims
The aim of this project was to validate the diagnostic abilities of TRBCB1 antibody when used in conjunction with an existing T-cell antibody panel.
Our aim was to use a control group of patient samples and assess TRBC1 expression on both CD4+ and CD8+ T cells separately, and calculate the ratio of TRBC1 positive and negative αβ T cells.
30 cases submitted for an investigation of a T -cell lymphoproliferative disorder were then assessed for TCBC1 expression.
We report here on our experience with the TRBC1 antibody, from validation to real-life experience by direct comparison of TRBC1 expression with molecular clonality testing.
Methods
10 normal peripheral blood samples were utilised to determine the normal biological range of TRBC1 on T cells. 30 consecutive patient samples submitted for investigation of a lymphoproliferative disorder were evaluated in a blind fashion independent of molecular analysis findings. Assessment of T-cell antigens including CD2/CD3/CD4/CD5/CD7/CD8/CD16/CD26/CD45/CD56/TCR αβ/TCR γδ along with TRBC1 were completed. Results were then compared to TCR gene rearrangement patterns by PCR.
Results
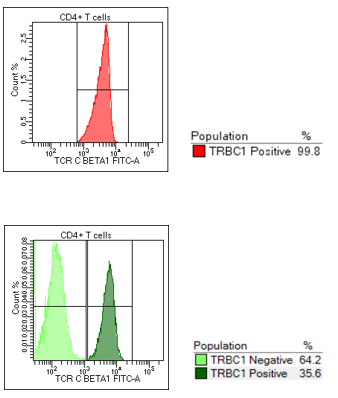
All normal control cases displayed a polytypic pattern of TRBC1 expression, with an average ratio of TRBC1 positive to TRBC1 negative expression of 1:2. Of the 30 consecutive cases evaluated, 7 cases were polytypic for TRBC1 and 22 cases were monotypic for TRBC1 expression. Direct comparison with molecular analysis demonstrated that no sample identified with monotypic (restricted) expression of TRBC1 was reported as polyclonal by PCR. A concordance rate of 95% (21/22 cases) between a clonal TCR gene rearrangement PCR result and a monotypic TRBC1 expression was calculated.
Conclusion
We have found the anti-TCR C Beta 1 antibody to be of high diagnostic value for assessment of clonality in patients with a suspected T cell neoplasm when used in conjunction with our existing diagnostic panel.
Incorporation of the TRBC1 antibody as part of the evaluation of a suspected T-cell neoplasm improves the identification of clonal T cells by flow cytometry and correlates well with molecular methods.
The importance of implementing a gating strategy directed at the abnormal population of T cells cannot be overstated.
This antibody is designed to detect clonal abnormalities in the constant region of the Beta chain of the TCR, therefore, it is not applicable for the assessment of normal γδ T cells or γδ T cell neoplasms.
To conclude, our results prove that this antibody improves the identification of clonal T cells by flow cytometry and is amenable to integration into a busy flow cytometry laboratory. By recognising the pitfalls and remaining mindful of the importance of gating strategies in the execution of this assay we find the TRBC1 antibody represents an excellent addition to the investigation of T-lymphoproliferative disorders by flow cytometry.
Keyword(s): Clonality, Flow cytometry, T cell lymphoma
Abstract: EP885
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
T-cell lymphomas are a pathologically and clinically complex group of heterogenous disorders. Often the interpretation of flow cytometry patterns may present challenges. Identification of an antibody specific for the TCR β constant region 1 (TRBC1) proposes to identify T-cell clonality and distinguish pathological T cells from normal T cells.
Aims
The aim of this project was to validate the diagnostic abilities of TRBCB1 antibody when used in conjunction with an existing T-cell antibody panel.
Our aim was to use a control group of patient samples and assess TRBC1 expression on both CD4+ and CD8+ T cells separately, and calculate the ratio of TRBC1 positive and negative αβ T cells.
30 cases submitted for an investigation of a T -cell lymphoproliferative disorder were then assessed for TCBC1 expression.
We report here on our experience with the TRBC1 antibody, from validation to real-life experience by direct comparison of TRBC1 expression with molecular clonality testing.
Methods
10 normal peripheral blood samples were utilised to determine the normal biological range of TRBC1 on T cells. 30 consecutive patient samples submitted for investigation of a lymphoproliferative disorder were evaluated in a blind fashion independent of molecular analysis findings. Assessment of T-cell antigens including CD2/CD3/CD4/CD5/CD7/CD8/CD16/CD26/CD45/CD56/TCR αβ/TCR γδ along with TRBC1 were completed. Results were then compared to TCR gene rearrangement patterns by PCR.
Results
All normal control cases displayed a polytypic pattern of TRBC1 expression, with an average ratio of TRBC1 positive to TRBC1 negative expression of 1:2. Of the 30 consecutive cases evaluated, 7 cases were polytypic for TRBC1 and 22 cases were monotypic for TRBC1 expression. Direct comparison with molecular analysis demonstrated that no sample identified with monotypic (restricted) expression of TRBC1 was reported as polyclonal by PCR. A concordance rate of 95% (21/22 cases) between a clonal TCR gene rearrangement PCR result and a monotypic TRBC1 expression was calculated.
Conclusion
We have found the anti-TCR C Beta 1 antibody to be of high diagnostic value for assessment of clonality in patients with a suspected T cell neoplasm when used in conjunction with our existing diagnostic panel.
Incorporation of the TRBC1 antibody as part of the evaluation of a suspected T-cell neoplasm improves the identification of clonal T cells by flow cytometry and correlates well with molecular methods.
The importance of implementing a gating strategy directed at the abnormal population of T cells cannot be overstated.
This antibody is designed to detect clonal abnormalities in the constant region of the Beta chain of the TCR, therefore, it is not applicable for the assessment of normal γδ T cells or γδ T cell neoplasms.
To conclude, our results prove that this antibody improves the identification of clonal T cells by flow cytometry and is amenable to integration into a busy flow cytometry laboratory. By recognising the pitfalls and remaining mindful of the importance of gating strategies in the execution of this assay we find the TRBC1 antibody represents an excellent addition to the investigation of T-lymphoproliferative disorders by flow cytometry.
Keyword(s): Clonality, Flow cytometry, T cell lymphoma