
Contributions
Abstract: EP882
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
The mechanisms of anti-tumor immune responses are currently under intense investigation in the era of immunotherapy. Cytosolic DNA of either endogenous or exogenous origin is sensed by the cGAS-STING pathway. Cyclic GMP-AMP (cGAMP) synthase (cGAS) is a cytosolic DNA sensor that activates anti-tumor immune responses through production of the second messenger cGAMP, which activates the adaptor protein STING. The latter then activates the transcription factors IRF3 and NF-κB via TBK1 and IKK kinases, respectively, inducing the expression of certain interferons (IFNs), such as IFN-alpha and IFN-beta, along with cytokines, which lead to cytotoxic T-cell mediated tumor cell killing. The expression patterns of STING protein in neoplastic cells of non Hodgkin lymphomas (NHL) is unknown to date.
Aims
To investigate the expression patterns and clinical significance of STING in T- and NK-cell lymphomas.
Methods
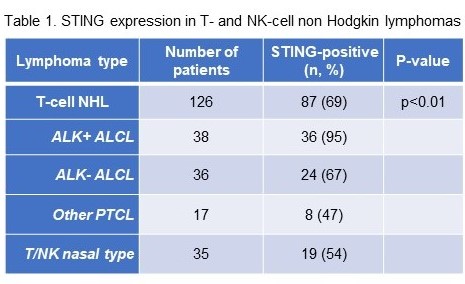
In the present study, we investigated STING expression and activation in 15 lymphoma cell lines by Western blot analysis using whole cell lysates and by immunohistochemistry (IHC) on formalin-fixed, paraffin-embedded cell blocks. The in vitro panel included four cell lines of ALK+ anaplastic large cell lymphoma (ALK+ ALCL) (Karpas 299, DEL, SUPM2, L-82), two of ALK- anaplastic large cell lymphoma (ALK- ALCL) (Mac1, Mac2a), one of Sezary syndrome (Hut78), and one of T-acute lymphoblastic leukemia (Jurkat), as well as eight B-cell lymphoma cell lines for comparison. In addition, STING protein expression was assessed in well-characterized cohorts of patients with T-cell NHLs using diagnostic biopsies obtained prior to treatment, immunohistochemical methods and a well validated monoclonal antibody. The T-cell NHL group (n=126) included 38 ALK+ ALCL, 36 ALK- ALCL, 17 other peripheral T-cell lymphomas (PTCLs) and 35 extranodal NK/ T-cell lymphomas of nasal type (NK/T-Nasal).
Results
High STING expression and activation assessed by Western blot analysis was found in all ALK+ and ALK- ALCL cell lines, as compared to Hut78 and Jurkat cells. Mac2a cells derived from a later stage of ALK- ALCL expressed higher levels of STING than Mac1 cells, which are derived from the same patient as Mac2a but at an earlier stage of disease. By contrast, the B-cell lymphoma cell lines were all negative or expressed very low levels of STING protein. Using a 10% cut-off for positivity, STING was differentially expressed among T-cell NHLs (Table 1, p<0.01, chi-square test) with ALK+ ALCL showing the highest frequency of STING-positive tumors. STING expression did not correlate with any clinical parameters including age, gender, Ann Arbor stage, IPI score, LDH levels, B-symptoms, anemia, and others. STING expression in the lymphoma cells showed a statistical trend towards inferior overall survival in the ALK- ALCL group but not in other PTCL subtypes.
Conclusion
STING is commonly and differentially expressed among T- and NK-cell NHL types and, therefore, pharmacologic modulation of cGAS-STING pathway activity may have therapeutic implications in T-cell malignancies.
Keyword(s): Immune response, Immunohistochemistry, T cell lymphoma
Abstract: EP882
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
The mechanisms of anti-tumor immune responses are currently under intense investigation in the era of immunotherapy. Cytosolic DNA of either endogenous or exogenous origin is sensed by the cGAS-STING pathway. Cyclic GMP-AMP (cGAMP) synthase (cGAS) is a cytosolic DNA sensor that activates anti-tumor immune responses through production of the second messenger cGAMP, which activates the adaptor protein STING. The latter then activates the transcription factors IRF3 and NF-κB via TBK1 and IKK kinases, respectively, inducing the expression of certain interferons (IFNs), such as IFN-alpha and IFN-beta, along with cytokines, which lead to cytotoxic T-cell mediated tumor cell killing. The expression patterns of STING protein in neoplastic cells of non Hodgkin lymphomas (NHL) is unknown to date.
Aims
To investigate the expression patterns and clinical significance of STING in T- and NK-cell lymphomas.
Methods
In the present study, we investigated STING expression and activation in 15 lymphoma cell lines by Western blot analysis using whole cell lysates and by immunohistochemistry (IHC) on formalin-fixed, paraffin-embedded cell blocks. The in vitro panel included four cell lines of ALK+ anaplastic large cell lymphoma (ALK+ ALCL) (Karpas 299, DEL, SUPM2, L-82), two of ALK- anaplastic large cell lymphoma (ALK- ALCL) (Mac1, Mac2a), one of Sezary syndrome (Hut78), and one of T-acute lymphoblastic leukemia (Jurkat), as well as eight B-cell lymphoma cell lines for comparison. In addition, STING protein expression was assessed in well-characterized cohorts of patients with T-cell NHLs using diagnostic biopsies obtained prior to treatment, immunohistochemical methods and a well validated monoclonal antibody. The T-cell NHL group (n=126) included 38 ALK+ ALCL, 36 ALK- ALCL, 17 other peripheral T-cell lymphomas (PTCLs) and 35 extranodal NK/ T-cell lymphomas of nasal type (NK/T-Nasal).
Results
High STING expression and activation assessed by Western blot analysis was found in all ALK+ and ALK- ALCL cell lines, as compared to Hut78 and Jurkat cells. Mac2a cells derived from a later stage of ALK- ALCL expressed higher levels of STING than Mac1 cells, which are derived from the same patient as Mac2a but at an earlier stage of disease. By contrast, the B-cell lymphoma cell lines were all negative or expressed very low levels of STING protein. Using a 10% cut-off for positivity, STING was differentially expressed among T-cell NHLs (Table 1, p<0.01, chi-square test) with ALK+ ALCL showing the highest frequency of STING-positive tumors. STING expression did not correlate with any clinical parameters including age, gender, Ann Arbor stage, IPI score, LDH levels, B-symptoms, anemia, and others. STING expression in the lymphoma cells showed a statistical trend towards inferior overall survival in the ALK- ALCL group but not in other PTCL subtypes.

Conclusion
STING is commonly and differentially expressed among T- and NK-cell NHL types and, therefore, pharmacologic modulation of cGAS-STING pathway activity may have therapeutic implications in T-cell malignancies.
Keyword(s): Immune response, Immunohistochemistry, T cell lymphoma


