
Contributions
Abstract: EP879
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
Lenalidomide (LEN) is an immunomodulatory drug with direct cytotoxic effects on tumor cells and stimulating effects on T and natural killer (NK) cells, making it an attractive combination partner for antibody therapies. However, clinical studies evaluating the addition of LEN to anti-CD20 based therapies compared to the current first-line standard of care (R-CHOP) for patients with diffuse large B-cell lymphoma (DLBCL) showed discordant results (ECOG‐ACRIN trial 1412; ROBUST study). Recently, the combination of LEN with the anti-CD19 antibody tafasitamab has shown clinically meaningful efficacy in patients with relapsed or refractory DLBCL (L-MIND study).
Aims
We aimed to investigate whether LEN treatment affects antigen levels of CD19 and CD20 on DLBCL cell lines from different subtypes and whether potential target antigen modulation has an influence on antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP).
Methods
CD19 and CD20 surface expression was analyzed in 7 DLBCL cell lines (3 activated B-cell, ABC; 3 germinal center B-cell, GCB; 1 unclassified) after incubation with 1/10 µM LEN for 24–96 hours (h). For ADCP assays, DLBCL target cells were pretreated with 10 µM LEN or dimethyl sulfoxide (DMSO) for 96 h and then incubated with macrophages at an effector to target (E:T) ratio of 2:1 and 10 nM tafasitamab or rituximab (RTX) for 3 h. For ADCC assays, DLBCL target cells were pretreated with 10 µM LEN or DMSO for 72 h and then incubated with NK cells at E:T ratios of 0.3:1 or 1:1 for 2 h in the presence of 1 nM tafasitamab, RTX or obinutuzumab (OBI). Antigen expression, ADCC activity, and ADCP activity were assessed by flow cytometry.
Results
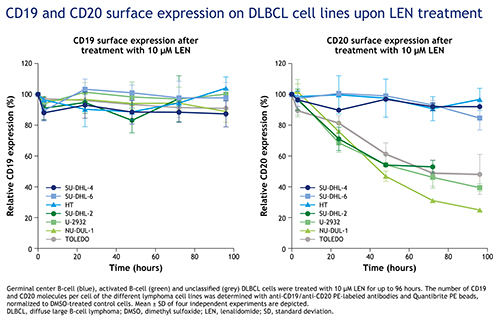
While CD19 expression on lymphoma cells was unaffected by LEN treatment, CD20 expression was significantly reduced by 47–75% in all cell lines from the ABC and unclassified DLBCL subtype (SU-DHL-2, U-2932, NU-DUL-1 and TOLEDO; Figure). CD20 surface expression of the tested GCB lines was not affected by LEN treatment.
In ADCP assays, decreased CD20 levels were associated with significantly diminished phagocytosis rates mediated by RTX in 3/7 cell lines (% relative reduction in phagocytosis: 37, 23, and 21 in SU-DHL-2, U-2932, and TOLEDO cells, respectively). In contrast, no or negligible reduction of RTX-mediated phagocytosis by LEN was observed in the remaining 4/7 cell lines. ADCP activity of the CD19-targeting antibody tafasitamab remained unchanged in all tested cell lines.
Significant reductions in target cell killing were also observed in ADCC assays with 2/3 LEN-treated lymphoma cell lines for RTX (% relative reduction in ADCC: 14 and 16 in SU-DHL-2 and U-2932 cells, respectively) and for the glyco-engineered antibody OBI (% relative reduction in ADCC: 13 and 22 in SU-DHL-2 and U-2932 cells respectively). By contrast, RTX-/OBI-mediated cytotoxicity was not reduced by LEN in the GCB cell line SU-DHL-4. ADCC activity of tafasitamab against all 3 tumor cell lines was not decreased by LEN pretreatment.
Conclusion
LEN treatment decreased surface expression of CD20 but not CD19 in all tested ABC and unclassified DLBCL cell lines. Rates of ADCC and ADCP with CD20 antibodies were reduced in 3/4 of these cell lines, but this was not observed with tafasitamab. These findings suggest that antigen expression levels could have an impact on combination therapies with LEN in certain DLBCL subtypes and support the rationale for combining tafasitamab with LEN, independent of cell of origin.
Keyword(s): CD20, DLBCL, Monoclonal antibody, Rituximab
Abstract: EP879
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
Lenalidomide (LEN) is an immunomodulatory drug with direct cytotoxic effects on tumor cells and stimulating effects on T and natural killer (NK) cells, making it an attractive combination partner for antibody therapies. However, clinical studies evaluating the addition of LEN to anti-CD20 based therapies compared to the current first-line standard of care (R-CHOP) for patients with diffuse large B-cell lymphoma (DLBCL) showed discordant results (ECOG‐ACRIN trial 1412; ROBUST study). Recently, the combination of LEN with the anti-CD19 antibody tafasitamab has shown clinically meaningful efficacy in patients with relapsed or refractory DLBCL (L-MIND study).
Aims
We aimed to investigate whether LEN treatment affects antigen levels of CD19 and CD20 on DLBCL cell lines from different subtypes and whether potential target antigen modulation has an influence on antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP).
Methods
CD19 and CD20 surface expression was analyzed in 7 DLBCL cell lines (3 activated B-cell, ABC; 3 germinal center B-cell, GCB; 1 unclassified) after incubation with 1/10 µM LEN for 24–96 hours (h). For ADCP assays, DLBCL target cells were pretreated with 10 µM LEN or dimethyl sulfoxide (DMSO) for 96 h and then incubated with macrophages at an effector to target (E:T) ratio of 2:1 and 10 nM tafasitamab or rituximab (RTX) for 3 h. For ADCC assays, DLBCL target cells were pretreated with 10 µM LEN or DMSO for 72 h and then incubated with NK cells at E:T ratios of 0.3:1 or 1:1 for 2 h in the presence of 1 nM tafasitamab, RTX or obinutuzumab (OBI). Antigen expression, ADCC activity, and ADCP activity were assessed by flow cytometry.
Results
While CD19 expression on lymphoma cells was unaffected by LEN treatment, CD20 expression was significantly reduced by 47–75% in all cell lines from the ABC and unclassified DLBCL subtype (SU-DHL-2, U-2932, NU-DUL-1 and TOLEDO; Figure). CD20 surface expression of the tested GCB lines was not affected by LEN treatment.
In ADCP assays, decreased CD20 levels were associated with significantly diminished phagocytosis rates mediated by RTX in 3/7 cell lines (% relative reduction in phagocytosis: 37, 23, and 21 in SU-DHL-2, U-2932, and TOLEDO cells, respectively). In contrast, no or negligible reduction of RTX-mediated phagocytosis by LEN was observed in the remaining 4/7 cell lines. ADCP activity of the CD19-targeting antibody tafasitamab remained unchanged in all tested cell lines.
Significant reductions in target cell killing were also observed in ADCC assays with 2/3 LEN-treated lymphoma cell lines for RTX (% relative reduction in ADCC: 14 and 16 in SU-DHL-2 and U-2932 cells, respectively) and for the glyco-engineered antibody OBI (% relative reduction in ADCC: 13 and 22 in SU-DHL-2 and U-2932 cells respectively). By contrast, RTX-/OBI-mediated cytotoxicity was not reduced by LEN in the GCB cell line SU-DHL-4. ADCC activity of tafasitamab against all 3 tumor cell lines was not decreased by LEN pretreatment.

Conclusion
LEN treatment decreased surface expression of CD20 but not CD19 in all tested ABC and unclassified DLBCL cell lines. Rates of ADCC and ADCP with CD20 antibodies were reduced in 3/4 of these cell lines, but this was not observed with tafasitamab. These findings suggest that antigen expression levels could have an impact on combination therapies with LEN in certain DLBCL subtypes and support the rationale for combining tafasitamab with LEN, independent of cell of origin.
Keyword(s): CD20, DLBCL, Monoclonal antibody, Rituximab


