
Contributions
Abstract: EP864
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
N6-methyladenosine (m6A) is the most abundant form of internal modifications in eukaryotic cells. m6A methylation is dynamically modulated by diverse types of regulators, including methyltransferases (‘writers’), RNA binding proteins (‘readers’), and demethylases (‘erasers’). Growing evidence has shown that m6A methylation plays essential role in the development and progression of multiple cancers. However, the functions of m6A methylation in diffuse large B-cell lymphoma (DLBCL) remains undefined.
Aims
In the study, we aimed to identify novel prognostic biomarker by m6A methylation regulators and explore its underlying mechanism in DLBCL.
Methods
The available expression data and the clinical information of DLBCL samples and normal samples were extracted from three databases. The expression level of the m6A methylation regulators was analyzed and the LASSO Cox regression was employed to calculate risk score. Kaplan-Meier survival analysis, univariate and multivariate Cox regression analyses, and ROC curve analysis were conducted. GO and KEGG enrichement were applied to explore the potential function. The lymph node biopsies of DLBCL patients and reactive hyperplasia cases were collected with informed consent to detect the expression of KIAA1429.
Results
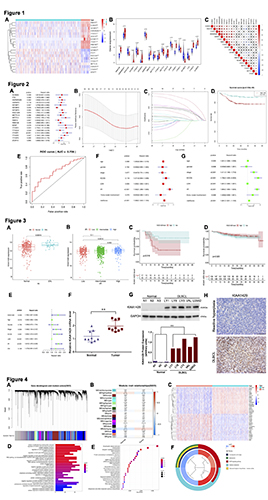
We firstly assessed the expression of m6A methylation regulators in DLBCL and found that most of them were dysregulated (p<0.001; Figure 1A-B). Subsequently, we discovered that the proportion of diverse m6A RNA methylation regulators was weakly to strongly relevant (p<0.05; Figure 1C).
The univariate Cox regression analysis indicated that six genes were high-risk and were significantly associated with OS (p<0.05, HR>1; Figure 2A), including KIAA1429 (p=0.043, HR=1.743). Six genes were selected based on the minimum criteria of LASSO Cox regression to establish the risk signature (Figure 2B-C). The high-risk group had a significantly shorter OS in DLBCL patients (p<0.001; Figure 2D). Furthermore, ROC curve, univariate, and multivariate Cox regression analyses showed that high m6A risk score acted as an independent indicator in DLBCL patients (p<0.001; Figure 2E-G).
KIAA1429 was found to be significantly associated with the IPI and DHL (p<0.05; Figure 3A-B). High expression of KIAA1429 resulted in a negative correlation with OS in DHL patients (p=0.018; Figure 3C). However, no significant difference was found in the OS of non-DHL patients (Figure 3D). Univariate analyses indicated that KIAA1429 was an independent indicator in DLBCL patients (p=0.04; Figure 3E). Enhanced expression levels of KIAA1429 mRNA and protein were verified in DLBCL cell lines (Figure 3F-G). Additionally, samples from DLBCL patients also showed significantly high expression of KIAA1429 compared to the reactive hyperplasia group (Figure 3H).
WGCNA was used to investigate the underlying mechanism of KIAA1429 (Figure 4A-B). A significant association between KIAA1429 expression and its module genes was identified (Figure 4C). GO and KEGG enrichment illuminated that KIAA1429 may act as a potential prognostic biomarker by regulating the mRNA processing and MAPK signaling pathways (Figure 4D-F)
Conclusion
In summary, we identified for the first time that m6A methylation regulators were dysregulated in DLBCL. More importantly, our study demonstrated the prognostic value of KIAA1429 in DHL patients. Further investigations on the mechanism of KIAA1429 in DLBCL may assist clinicians in achieving individualized treatment for this patient population.
Keyword(s): Diffuse large B cell lymphoma, Prognosis
Abstract: EP864
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
N6-methyladenosine (m6A) is the most abundant form of internal modifications in eukaryotic cells. m6A methylation is dynamically modulated by diverse types of regulators, including methyltransferases (‘writers’), RNA binding proteins (‘readers’), and demethylases (‘erasers’). Growing evidence has shown that m6A methylation plays essential role in the development and progression of multiple cancers. However, the functions of m6A methylation in diffuse large B-cell lymphoma (DLBCL) remains undefined.
Aims
In the study, we aimed to identify novel prognostic biomarker by m6A methylation regulators and explore its underlying mechanism in DLBCL.
Methods
The available expression data and the clinical information of DLBCL samples and normal samples were extracted from three databases. The expression level of the m6A methylation regulators was analyzed and the LASSO Cox regression was employed to calculate risk score. Kaplan-Meier survival analysis, univariate and multivariate Cox regression analyses, and ROC curve analysis were conducted. GO and KEGG enrichement were applied to explore the potential function. The lymph node biopsies of DLBCL patients and reactive hyperplasia cases were collected with informed consent to detect the expression of KIAA1429.
Results
We firstly assessed the expression of m6A methylation regulators in DLBCL and found that most of them were dysregulated (p<0.001; Figure 1A-B). Subsequently, we discovered that the proportion of diverse m6A RNA methylation regulators was weakly to strongly relevant (p<0.05; Figure 1C).
The univariate Cox regression analysis indicated that six genes were high-risk and were significantly associated with OS (p<0.05, HR>1; Figure 2A), including KIAA1429 (p=0.043, HR=1.743). Six genes were selected based on the minimum criteria of LASSO Cox regression to establish the risk signature (Figure 2B-C). The high-risk group had a significantly shorter OS in DLBCL patients (p<0.001; Figure 2D). Furthermore, ROC curve, univariate, and multivariate Cox regression analyses showed that high m6A risk score acted as an independent indicator in DLBCL patients (p<0.001; Figure 2E-G).
KIAA1429 was found to be significantly associated with the IPI and DHL (p<0.05; Figure 3A-B). High expression of KIAA1429 resulted in a negative correlation with OS in DHL patients (p=0.018; Figure 3C). However, no significant difference was found in the OS of non-DHL patients (Figure 3D). Univariate analyses indicated that KIAA1429 was an independent indicator in DLBCL patients (p=0.04; Figure 3E). Enhanced expression levels of KIAA1429 mRNA and protein were verified in DLBCL cell lines (Figure 3F-G). Additionally, samples from DLBCL patients also showed significantly high expression of KIAA1429 compared to the reactive hyperplasia group (Figure 3H).
WGCNA was used to investigate the underlying mechanism of KIAA1429 (Figure 4A-B). A significant association between KIAA1429 expression and its module genes was identified (Figure 4C). GO and KEGG enrichment illuminated that KIAA1429 may act as a potential prognostic biomarker by regulating the mRNA processing and MAPK signaling pathways (Figure 4D-F)

Conclusion
In summary, we identified for the first time that m6A methylation regulators were dysregulated in DLBCL. More importantly, our study demonstrated the prognostic value of KIAA1429 in DHL patients. Further investigations on the mechanism of KIAA1429 in DLBCL may assist clinicians in achieving individualized treatment for this patient population.
Keyword(s): Diffuse large B cell lymphoma, Prognosis


