Contributions
Abstract: EP862
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
Glycoprotein prostaglandin D2 Synthase (PTGDS) is a member of lipocalin superfamily and plays a dual role in catalyzing the conversion of PGH2 to PGD2 and transporting lipophilic substances. AT56 is a selective inhibitor of PTGDS. PTGDS has been involved in several cellular processes including cancer, yet its role in carcinogenesis is contradictory.
Aims
Herein, we aim to investigate the functional significance of PTGDS in DLBCL and propose a novel therapeutic strategy for DLBCL.
Methods
Peripheral blood mononuclear cells were obtained from healthy volunteers. Lymph node biopsies from 53 DLBCL patients and 28 reactive hyperplasia cases were collected with informed consents. CD19+ B cells were purified by CD19+ magnetic microbeads. Expression level of PTGDS was detected by western blotting and ELISA. Lentivirus vectors were transfected to stably knockdown or overexpress PTGDS. CoIP and mass spectrometry were performed to investigate molecular mechanism. Point mutations were created to verify glycosylation sites. BALB/c nude mice were subcutaneously injected with DLBCL cells and mice were imaged using In-Vivo small animal imaging system. p < 0.05 was considered statistically significant.
Results
Upregulation of PTGDS mRNA in DLBCL was identified based on Oncomine database. The expression level of PTGDS protein was high in DLBCL tissues and serum, which was associated with poor prognosis. High expression of PTGDS protein was also confirmed in DLBCL cells.
PTGDS overexpression and rhPTGDS were found to promote cell proliferation. PTGDS knockdown significantly restrained cell proliferation, promoted cell cycle arrest and cell apoptosis. AT56 suppressed cell proliferation, induced G0/G1 phase arrest and increased cell apoptosis in a dose- and time-dependent manner. AT56 also enhanced sensitivity to Bendamustine and Adriamycin. Besides, in vivo studies indicated PTGDS knockdown and AT56 exerted anti-tumor effect in DLBCL.
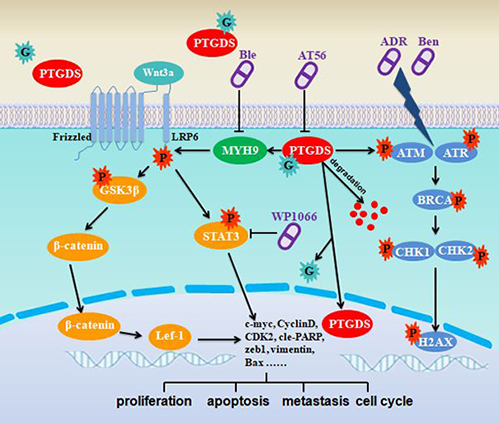
CoIP and mass spectrometry indicated PTGDS interacted with oncogenic factor, MYH9. PTGDS inhibition reduced expression of MYH9 and inhibited activation of Wnt-β-catenin/STAT3 pathway. Moreover, Wnt3a rescued the inhibitory effect of AT56 on malignant behaviors, including cell proliferation, cell cycle arrest and cell apoptosis. Besides, the role of AT56 on Wnt pathway could be reversed by Wnt3a. The proliferation inhibition and apoptosis induction caused by AT56 were enhanced by WP1066 while WP1066 reversed proliferation promotion caused by PTGDS overexpression. The enhanced proliferation by PTGDS overexpression was restored by Blebbistatin and it restored the activation of Wnt-β-catenin/STAT3 pathway caused by PTGDS overexpression.
We confirmed the N-glycoprotein of PTGDS protein in Asn 51 and Asn78 via point mutation and the abnormal glycosylation resulted in nuclear translocation, prolonged half time and enhanced oncogenic role.
Conclusion
Our investigations identified for the first time the oncogenic role of PTGDS in DLBCL tumorigenesis. Expression of PTGDS was upregulated and associated with prognosis of DLBCL patients. Besides, in vitro and in vivo studies indicated that PTGDS knockdown and AT56 treatment exerted anti-tumor effect through regulating through MYH9-Wnt-β-catenin/STAT3 axis. This study suggests that PTGDS could be a potential molecular target for DLBCL treatment and highlights the potential role of AT56 as a novel therapeutic strategy for DLBCL.
Keyword(s): Diffuse large B cell lymphoma, Inhibitor, Prognosis, Wnt
Abstract: EP862
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
Glycoprotein prostaglandin D2 Synthase (PTGDS) is a member of lipocalin superfamily and plays a dual role in catalyzing the conversion of PGH2 to PGD2 and transporting lipophilic substances. AT56 is a selective inhibitor of PTGDS. PTGDS has been involved in several cellular processes including cancer, yet its role in carcinogenesis is contradictory.
Aims
Herein, we aim to investigate the functional significance of PTGDS in DLBCL and propose a novel therapeutic strategy for DLBCL.
Methods
Peripheral blood mononuclear cells were obtained from healthy volunteers. Lymph node biopsies from 53 DLBCL patients and 28 reactive hyperplasia cases were collected with informed consents. CD19+ B cells were purified by CD19+ magnetic microbeads. Expression level of PTGDS was detected by western blotting and ELISA. Lentivirus vectors were transfected to stably knockdown or overexpress PTGDS. CoIP and mass spectrometry were performed to investigate molecular mechanism. Point mutations were created to verify glycosylation sites. BALB/c nude mice were subcutaneously injected with DLBCL cells and mice were imaged using In-Vivo small animal imaging system. p < 0.05 was considered statistically significant.
Results
Upregulation of PTGDS mRNA in DLBCL was identified based on Oncomine database. The expression level of PTGDS protein was high in DLBCL tissues and serum, which was associated with poor prognosis. High expression of PTGDS protein was also confirmed in DLBCL cells.
PTGDS overexpression and rhPTGDS were found to promote cell proliferation. PTGDS knockdown significantly restrained cell proliferation, promoted cell cycle arrest and cell apoptosis. AT56 suppressed cell proliferation, induced G0/G1 phase arrest and increased cell apoptosis in a dose- and time-dependent manner. AT56 also enhanced sensitivity to Bendamustine and Adriamycin. Besides, in vivo studies indicated PTGDS knockdown and AT56 exerted anti-tumor effect in DLBCL.
CoIP and mass spectrometry indicated PTGDS interacted with oncogenic factor, MYH9. PTGDS inhibition reduced expression of MYH9 and inhibited activation of Wnt-β-catenin/STAT3 pathway. Moreover, Wnt3a rescued the inhibitory effect of AT56 on malignant behaviors, including cell proliferation, cell cycle arrest and cell apoptosis. Besides, the role of AT56 on Wnt pathway could be reversed by Wnt3a. The proliferation inhibition and apoptosis induction caused by AT56 were enhanced by WP1066 while WP1066 reversed proliferation promotion caused by PTGDS overexpression. The enhanced proliferation by PTGDS overexpression was restored by Blebbistatin and it restored the activation of Wnt-β-catenin/STAT3 pathway caused by PTGDS overexpression.
We confirmed the N-glycoprotein of PTGDS protein in Asn 51 and Asn78 via point mutation and the abnormal glycosylation resulted in nuclear translocation, prolonged half time and enhanced oncogenic role.
Conclusion
Our investigations identified for the first time the oncogenic role of PTGDS in DLBCL tumorigenesis. Expression of PTGDS was upregulated and associated with prognosis of DLBCL patients. Besides, in vitro and in vivo studies indicated that PTGDS knockdown and AT56 treatment exerted anti-tumor effect through regulating through MYH9-Wnt-β-catenin/STAT3 axis. This study suggests that PTGDS could be a potential molecular target for DLBCL treatment and highlights the potential role of AT56 as a novel therapeutic strategy for DLBCL.
Keyword(s): Diffuse large B cell lymphoma, Inhibitor, Prognosis, Wnt