
Contributions
Abstract: EP860
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
Despite immunochemotherapy provides durable responses in follicular lymphoma (FL) patients, the majority of them eventually relapse. Minimal residual disease (MRD) analysis, based on the detection of Bcl-2/IGH rearrangement by highly sensitive polymerase-chain reaction (PCR), is a standardized approach, able to early identify patients at risk of disease recurrence. Nevertheless, this tool has some limitations, including a 40% failure of marker identification. Next-generation sequencing (NGS) is going to overcome these issues, possibly increasing the number of patients eligible for MRD.
Aims
To screen by NGS a cohort of 120 FL who failed Bcl-2/IGH marker identification by standardized EuroMRD PCR, enrolled in the FIL 'FOLL12' prospective clinical trial (EudraCT: 2012-003170-60), in order to identify new markers for MRD tracking during the follow-up (FU). First results of marker identification are here described, while complete MRD results will be presented elsewhere.
Methods
Advanced FL received upfront R-CHOP or BR, followed by a combined PET/MRD response-based post-induction therapy vs conventional maintenance with rituximab. Bone marrow (BM) samples were centralized at the Italian Network laboratories for MRD analysis. Baseline molecular evaluation was available for 615/628 cases who achieved PET negativity at end of induction. 270 of them (44%) lacked a conventional Bcl-2/IGH marker. Therefore, 163 (60%) were selected for BM lymphoma infiltration (median 25%, 1-90%), of whom 120 (74%) had available samples for analysis. Baseline gDNA was screened by IGH-NGS using EuroClonality-NGS framework region 1 (FR1) primers; IGH clonotypes identification was done by ARReST/Interrogate. In parallel, targeted locus amplification (TLA) on the IGH enhancer was used to identify novel Bcl-2/IGH rearrangements in a preliminary cohort of 15 patients. Finally, to test the reliability of newly identified markers, we generated patient-specific primers and carried out MRD analysis by ASO qPCR following EuroMRD guidelines in three cases.
Results
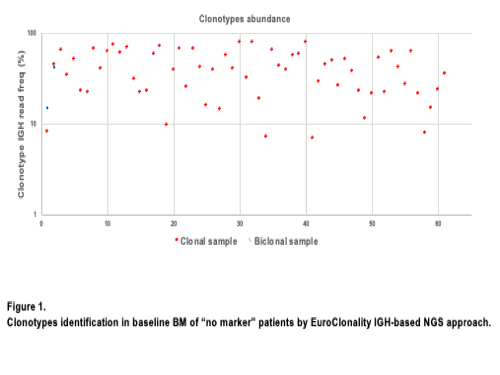
111/120 (93%) sequenced samples passed the quality control (>10000 raw reads). A clonotype (>5% of IGH-VDJ annotated reads) was identified in 56% (63/111): 61 monoclonal and 2 biclonal, 54 productive and 9 unproductive IGH rearrangements (Figure 1). Moreover, in 8/15 (53%) patients screened by TLA a novel Bcl-2/IGH rearrangement was found; interestingly, TLA was able to identify a marker in 2 out of 5 cases failed by IGH-NGS. Finally, the reliability of these markers for MRD detection was preliminary assessed in three patients by ASO qPCR (targeting one Bcl2/IGH and two IGH rearrangements). The assays reached high level of sensitivity (from 1E-04 to 5E-05) and MRD kinetics in FU well described patients’ outcome.
Conclusion
Preliminary data on wide NGS-based marker screening in FL patients lacking a conventional MRD marker in a clinical trial suggested that: 1) EuroClonality-NGS IGH approach was able to provide a new marker in more than half of the patients with BM infiltration; 2) TLA approach showed similar success rates in a small patients group (further experiments are ongoing); 3) the two techniques are not alternative and should rather be considered as complementary; 4) the new markers allowed to perform a “proof of concept” MRD monitoring by ASO qPCR, according to the strict EuroMRD rules. These data are highly promising to provide a MRD marker in the majority of FL patients: next steps of the present project will evaluate MRD by NGS in FU samples and assess its clinical impact.
Keyword(s): BCL2, Clinical trial, Immunoglobulin gene, MRD
Abstract: EP860
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
Despite immunochemotherapy provides durable responses in follicular lymphoma (FL) patients, the majority of them eventually relapse. Minimal residual disease (MRD) analysis, based on the detection of Bcl-2/IGH rearrangement by highly sensitive polymerase-chain reaction (PCR), is a standardized approach, able to early identify patients at risk of disease recurrence. Nevertheless, this tool has some limitations, including a 40% failure of marker identification. Next-generation sequencing (NGS) is going to overcome these issues, possibly increasing the number of patients eligible for MRD.
Aims
To screen by NGS a cohort of 120 FL who failed Bcl-2/IGH marker identification by standardized EuroMRD PCR, enrolled in the FIL 'FOLL12' prospective clinical trial (EudraCT: 2012-003170-60), in order to identify new markers for MRD tracking during the follow-up (FU). First results of marker identification are here described, while complete MRD results will be presented elsewhere.
Methods
Advanced FL received upfront R-CHOP or BR, followed by a combined PET/MRD response-based post-induction therapy vs conventional maintenance with rituximab. Bone marrow (BM) samples were centralized at the Italian Network laboratories for MRD analysis. Baseline molecular evaluation was available for 615/628 cases who achieved PET negativity at end of induction. 270 of them (44%) lacked a conventional Bcl-2/IGH marker. Therefore, 163 (60%) were selected for BM lymphoma infiltration (median 25%, 1-90%), of whom 120 (74%) had available samples for analysis. Baseline gDNA was screened by IGH-NGS using EuroClonality-NGS framework region 1 (FR1) primers; IGH clonotypes identification was done by ARReST/Interrogate. In parallel, targeted locus amplification (TLA) on the IGH enhancer was used to identify novel Bcl-2/IGH rearrangements in a preliminary cohort of 15 patients. Finally, to test the reliability of newly identified markers, we generated patient-specific primers and carried out MRD analysis by ASO qPCR following EuroMRD guidelines in three cases.
Results
111/120 (93%) sequenced samples passed the quality control (>10000 raw reads). A clonotype (>5% of IGH-VDJ annotated reads) was identified in 56% (63/111): 61 monoclonal and 2 biclonal, 54 productive and 9 unproductive IGH rearrangements (Figure 1). Moreover, in 8/15 (53%) patients screened by TLA a novel Bcl-2/IGH rearrangement was found; interestingly, TLA was able to identify a marker in 2 out of 5 cases failed by IGH-NGS. Finally, the reliability of these markers for MRD detection was preliminary assessed in three patients by ASO qPCR (targeting one Bcl2/IGH and two IGH rearrangements). The assays reached high level of sensitivity (from 1E-04 to 5E-05) and MRD kinetics in FU well described patients’ outcome.

Conclusion
Preliminary data on wide NGS-based marker screening in FL patients lacking a conventional MRD marker in a clinical trial suggested that: 1) EuroClonality-NGS IGH approach was able to provide a new marker in more than half of the patients with BM infiltration; 2) TLA approach showed similar success rates in a small patients group (further experiments are ongoing); 3) the two techniques are not alternative and should rather be considered as complementary; 4) the new markers allowed to perform a “proof of concept” MRD monitoring by ASO qPCR, according to the strict EuroMRD rules. These data are highly promising to provide a MRD marker in the majority of FL patients: next steps of the present project will evaluate MRD by NGS in FU samples and assess its clinical impact.
Keyword(s): BCL2, Clinical trial, Immunoglobulin gene, MRD


