
Contributions
Abstract: EP854
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
Treatment failure after RCHOP is associated with poor outcome in DLBCL. Peripheral blood (PB) absolute monocyte (AMC) and lymphocyte (ALC) counts at diagnosis can predict outcome, but the mechanism is unknown.
Aims
To characterise and functionally define the PB immune compartment in DLBCL.
Methods
Full blood counts (FBC) from 116 healthy donors and 146 previously untreated DLBCL patients were assessed. Twenty cytokines were measured in duplicate in serum from healthy donors (HD, n=11) and DLBCL patients at diagnosis (n=64) using the mesoscale discovery electrochemiluminescence platform. Peripheral blood mononuclear cells (PBMCs) from HD (n=8) and DLBCL patients (n=42) were analysed by mass cytometry (MC) to phenotype immune cell populations in depth. An intracellular cytokine staining MC protocol was applied to a subset of these PBMC samples for functional assessment. A standard HD control sample was acquired with each MC batch. Normalised data were analysed using the OMIQ platform.
Results
The median AMC was higher and ALC lower in DLBCL (p<0.0001). The AMC / ALC prognostic score defined 3 groups with distinct outcome in DLBCL (p<0.0001). Eight of 20 cytokines analysed were elevated in DLBCL, with 5 of these cytokines (CCL3, TNF, IL1RA, IL6 and IL10, p<0.05) significantly higher at diagnosis in patients who subsequently had relapse/refractory disease post RCHOP (R/R) compared to those with maintained complete remission (CR). Cytokine levels were independent of cell of origin (COO) except for IL1RA which was elevated in non-GCB.
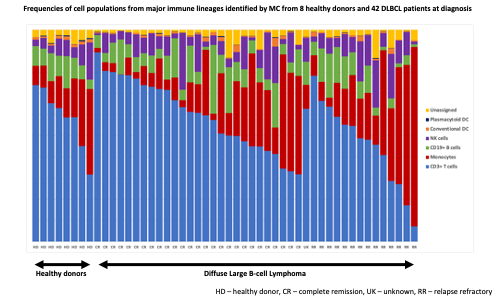
Gated immune populations identified by MC were assessed for significant differential abundance with edgeR (FDR<0.05). Intermediate and non-classical monocytes were higher in DLBCL, whereas CD4 effector memory (EM1) and CD8 central memory (CM) were higher in HD. Within DLBCL samples, non-classical monocytes and CD8 effector memory (EM3) were more abundant in patients with subsequent R/R disease. FlowSOM clustering followed by edgeR analysis revealed 3 monocyte clusters at diagnosis associated with disease relapse. The most differentiating cluster consisted of classical monocytes with a suppressor phenotype (CD14hi, HLA-DRlo) and expression of CD163 and CD184. The most differentiating T cell cluster associated with subsequent relapse had a CD8 EM3 phenotype. These findings were independent of COO.
Functional analysis revealed constitutive over production of CCL3 and IL6 in DLBCL, predominantly by CD14+ monocytes (p=0.0025). Stimulation with LPS demonstrated blunted cytokine production responses in DLBCL, most pronounced in cases that relapsed. Stimulation with PMA/inomycin demonstrated no evidence of PB T cell exhaustion, with hypersensitivity of TNF and IL2 production in DLBCL, predominantly by CD4 T cells. Activated granzyme B / perforin positive NK cells were increased in DLBCL.
Conclusion
We find distinct immune cell and cytokine signatures associated with DLBCL and with outcome following RCHOP. Specifically, we identify cytokine overproduction in DLBCL, the main PB source being CD14+ monocytes, suggesting a non-cell autonomous role for PB cytokine production in DLBCL. We identify immune populations by manual gating and FlowSOM clustering with differential abundance in DLBCL and in those cases that subsequently relapsed, reflecting immune dysfunction associated with disease and outcome. Our data provide an explanation for the prognostic nature of the AMC /ALC, support the challenge to identify biomarkers and provide insight into steps that may be required to overcome the immune defects in RCHOP refractory DLBCL.
Keyword(s): Cytokine, DLBCL, Lymphoma
Abstract: EP854
Type: E-Poster Presentation
Session title: Lymphoma Biology & Translational Research
Background
Treatment failure after RCHOP is associated with poor outcome in DLBCL. Peripheral blood (PB) absolute monocyte (AMC) and lymphocyte (ALC) counts at diagnosis can predict outcome, but the mechanism is unknown.
Aims
To characterise and functionally define the PB immune compartment in DLBCL.
Methods
Full blood counts (FBC) from 116 healthy donors and 146 previously untreated DLBCL patients were assessed. Twenty cytokines were measured in duplicate in serum from healthy donors (HD, n=11) and DLBCL patients at diagnosis (n=64) using the mesoscale discovery electrochemiluminescence platform. Peripheral blood mononuclear cells (PBMCs) from HD (n=8) and DLBCL patients (n=42) were analysed by mass cytometry (MC) to phenotype immune cell populations in depth. An intracellular cytokine staining MC protocol was applied to a subset of these PBMC samples for functional assessment. A standard HD control sample was acquired with each MC batch. Normalised data were analysed using the OMIQ platform.
Results
The median AMC was higher and ALC lower in DLBCL (p<0.0001). The AMC / ALC prognostic score defined 3 groups with distinct outcome in DLBCL (p<0.0001). Eight of 20 cytokines analysed were elevated in DLBCL, with 5 of these cytokines (CCL3, TNF, IL1RA, IL6 and IL10, p<0.05) significantly higher at diagnosis in patients who subsequently had relapse/refractory disease post RCHOP (R/R) compared to those with maintained complete remission (CR). Cytokine levels were independent of cell of origin (COO) except for IL1RA which was elevated in non-GCB.
Gated immune populations identified by MC were assessed for significant differential abundance with edgeR (FDR<0.05). Intermediate and non-classical monocytes were higher in DLBCL, whereas CD4 effector memory (EM1) and CD8 central memory (CM) were higher in HD. Within DLBCL samples, non-classical monocytes and CD8 effector memory (EM3) were more abundant in patients with subsequent R/R disease. FlowSOM clustering followed by edgeR analysis revealed 3 monocyte clusters at diagnosis associated with disease relapse. The most differentiating cluster consisted of classical monocytes with a suppressor phenotype (CD14hi, HLA-DRlo) and expression of CD163 and CD184. The most differentiating T cell cluster associated with subsequent relapse had a CD8 EM3 phenotype. These findings were independent of COO.
Functional analysis revealed constitutive over production of CCL3 and IL6 in DLBCL, predominantly by CD14+ monocytes (p=0.0025). Stimulation with LPS demonstrated blunted cytokine production responses in DLBCL, most pronounced in cases that relapsed. Stimulation with PMA/inomycin demonstrated no evidence of PB T cell exhaustion, with hypersensitivity of TNF and IL2 production in DLBCL, predominantly by CD4 T cells. Activated granzyme B / perforin positive NK cells were increased in DLBCL.

Conclusion
We find distinct immune cell and cytokine signatures associated with DLBCL and with outcome following RCHOP. Specifically, we identify cytokine overproduction in DLBCL, the main PB source being CD14+ monocytes, suggesting a non-cell autonomous role for PB cytokine production in DLBCL. We identify immune populations by manual gating and FlowSOM clustering with differential abundance in DLBCL and in those cases that subsequently relapsed, reflecting immune dysfunction associated with disease and outcome. Our data provide an explanation for the prognostic nature of the AMC /ALC, support the challenge to identify biomarkers and provide insight into steps that may be required to overcome the immune defects in RCHOP refractory DLBCL.
Keyword(s): Cytokine, DLBCL, Lymphoma


