
Contributions
Abstract: EP846
Type: E-Poster Presentation
Session title: Iron metabolism, deficiency and overload
Background
Hepcidin, the central regulator of systemic iron homeostasis, is synthesized predominantly in hepatocytes, and dysregulation of its production leads to a variety of life-limiting iron disorders. SLN124 is a liver-targeted N-Acetylgalactosamine (GalNAc)-conjugated siRNA which works by increasing hepcidin by silencing its repressor TMPRSS6. SLN124 is pharmacologically active in the mouse & cynomolgus monkey.
Aims
The primary objective was to evaluate the non-clinical safety and PK/PD of SLN124 in relevant toxicology species (mouse & cynomolgus monkey) to enable evaluation of SLN124 in the clinic.
Methods
In vitro serum stability (mouse, monkey & human) and metabolism (human S9 fraction/lysosomes and plasma) was evaluated. Potential genotoxicity was determined in in vitro micronucleus and mammalian cell gene mutation assays. Cytokine release was evaluated in vitro in whole blood assays (human & monkey) and in vivo (mouse & monkey). Potential immunogenicity was assessed in vivo in rabbits using LPH-conjugated SLN124. Biodistribution was considered in the mouse, following subcutaneous dosing at 10 mg/kg over 21 days. In vivo, SLN124 was evaluated in mouse & monkey repeat dose studies, the longest duration study was 13-weeks in both species, with a 12-week recovery period. Mice & monkeys were dosed subcutaneously at 0, 10, 30 & 83 mg/kg Q2Wx6 with weekly dosing for the last 2 doses. Population PK/PD modelling & simulation of the mouse and cynomolgus monkey SLN124 PK/PD was used to estimate an appropriate clinical starting dose and dose range.
Results
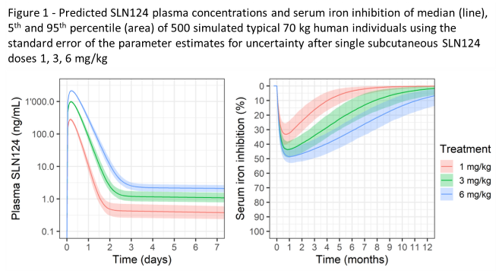
SLN124 is stable in serum, plasma and human S9 fraction/lysosomes. SLN124 was neither clastogenic nor mutagenic in the completed in vitro genotoxicity studies. No cytokine release was noted in the human whole blood assay or in vivo in the mouse & monkey. As rabbit immunisation did not result in any antibody generation, SLN124 does not pose an immunogenicity risk. SLN124 showed a typical GalNAc siRNA distribution pattern, with significant levels in the mouse liver and kidney only (peak levels noted after 6 hrs, with 25% remaining in the liver after 7 days with 6% by day 21). In the 13-week repeat dose studies, SLN124 was clinically well tolerated in the mouse & monkey, with expected non-adverse changes in the liver (reversible hepatocyte degeneration/regeneration in the mouse & basophilic granules in Kupffer cells in the monkey), kidney (reversible minor increased kidney weight and renal tubular basophilia/vacuolation in the mouse) and lymph nodes (vacuolated macrophages in the monkey). The NOAEL was ≥83 mg/kg for both the mouse & monkey (highest dose tested). Population PK/PD modelling of the observed SLN124 exposure in plasma & serum iron levels in the mouse and/or monkey was used to predict human exposure and serum iron inhibition in the clinic. At 1 mg/kg (~30 fold below the human equivalent dose of the monkey NOAEL), the model predicts a maximum 34% reduction in serum iron levels (Figure 1).
Conclusion
The non-clinical safety profile of SLN124 is consistent with the known GalNAc siRNA safety profile and supportive of clinical entry, with PK/PD modelling supporting 1 mg/kg as an appropriate safe starting dose. Two clinical studies are underway: NCT04559971 in healthy volunteers and NCT04718844 in non-transfusion dependent b-thalassaemia and VL and LR-MDS patients.
Keyword(s): Hepcidin, Iron
Abstract: EP846
Type: E-Poster Presentation
Session title: Iron metabolism, deficiency and overload
Background
Hepcidin, the central regulator of systemic iron homeostasis, is synthesized predominantly in hepatocytes, and dysregulation of its production leads to a variety of life-limiting iron disorders. SLN124 is a liver-targeted N-Acetylgalactosamine (GalNAc)-conjugated siRNA which works by increasing hepcidin by silencing its repressor TMPRSS6. SLN124 is pharmacologically active in the mouse & cynomolgus monkey.
Aims
The primary objective was to evaluate the non-clinical safety and PK/PD of SLN124 in relevant toxicology species (mouse & cynomolgus monkey) to enable evaluation of SLN124 in the clinic.
Methods
In vitro serum stability (mouse, monkey & human) and metabolism (human S9 fraction/lysosomes and plasma) was evaluated. Potential genotoxicity was determined in in vitro micronucleus and mammalian cell gene mutation assays. Cytokine release was evaluated in vitro in whole blood assays (human & monkey) and in vivo (mouse & monkey). Potential immunogenicity was assessed in vivo in rabbits using LPH-conjugated SLN124. Biodistribution was considered in the mouse, following subcutaneous dosing at 10 mg/kg over 21 days. In vivo, SLN124 was evaluated in mouse & monkey repeat dose studies, the longest duration study was 13-weeks in both species, with a 12-week recovery period. Mice & monkeys were dosed subcutaneously at 0, 10, 30 & 83 mg/kg Q2Wx6 with weekly dosing for the last 2 doses. Population PK/PD modelling & simulation of the mouse and cynomolgus monkey SLN124 PK/PD was used to estimate an appropriate clinical starting dose and dose range.
Results
SLN124 is stable in serum, plasma and human S9 fraction/lysosomes. SLN124 was neither clastogenic nor mutagenic in the completed in vitro genotoxicity studies. No cytokine release was noted in the human whole blood assay or in vivo in the mouse & monkey. As rabbit immunisation did not result in any antibody generation, SLN124 does not pose an immunogenicity risk. SLN124 showed a typical GalNAc siRNA distribution pattern, with significant levels in the mouse liver and kidney only (peak levels noted after 6 hrs, with 25% remaining in the liver after 7 days with 6% by day 21). In the 13-week repeat dose studies, SLN124 was clinically well tolerated in the mouse & monkey, with expected non-adverse changes in the liver (reversible hepatocyte degeneration/regeneration in the mouse & basophilic granules in Kupffer cells in the monkey), kidney (reversible minor increased kidney weight and renal tubular basophilia/vacuolation in the mouse) and lymph nodes (vacuolated macrophages in the monkey). The NOAEL was ≥83 mg/kg for both the mouse & monkey (highest dose tested). Population PK/PD modelling of the observed SLN124 exposure in plasma & serum iron levels in the mouse and/or monkey was used to predict human exposure and serum iron inhibition in the clinic. At 1 mg/kg (~30 fold below the human equivalent dose of the monkey NOAEL), the model predicts a maximum 34% reduction in serum iron levels (Figure 1).

Conclusion
The non-clinical safety profile of SLN124 is consistent with the known GalNAc siRNA safety profile and supportive of clinical entry, with PK/PD modelling supporting 1 mg/kg as an appropriate safe starting dose. Two clinical studies are underway: NCT04559971 in healthy volunteers and NCT04718844 in non-transfusion dependent b-thalassaemia and VL and LR-MDS patients.
Keyword(s): Hepcidin, Iron


