
Contributions
Abstract: EP845
Type: E-Poster Presentation
Session title: Iron metabolism, deficiency and overload
Background
The consecutive-day split dosing of iron supplements is the most common treatment of iron deficiency anemia (IDA) in clinical practice. Although recent ferrokinetic studies in iron-deficient young women demonstrated greater iron absorption with alternate-day single dosing compared to conventional twice-daily split dosing, there have been no studies evaluating long-term efficacy between these two schemes in treatment of IDA in reproductive-aged women.
Aims
This open-label, non-inferiority, randomized controlled trial (TCTR20200614001) primarily aims to evaluate efficacy and safety between alternate-day single dosing of iron supplements versus consecutive-day split dosing in reproductive-aged women with IDA.
Methods
Women aged 18-50 years were eligible for enrollment. IDA was defined by serum ferritin levels (sF) <30 µg/L. Patients randomized in alternate-day dosing were given single doses of ferrous sulfate 400 mg on alternate days, while patients in consecutive-day dosing were given ferrous sulfate 200 mg twice daily. The primary outcome was the proportion of patients with ≥ 2 g/dL hemoglobin (Hb) increase at day 30 (D30) post-treatment. The secondary outcomes were the proportion of patients with ≥ 1 g Hb increase at D30; Hb and sF at D30 and D90; and incidence of adverse events.
Results
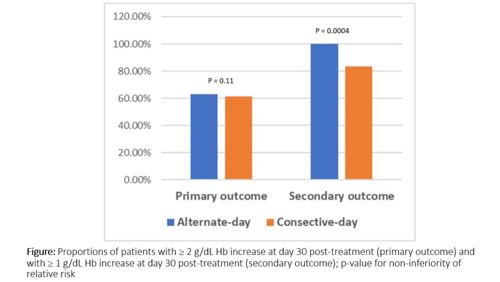
Of 37 in preliminary analysis, 19 and 18 were randomized to alternate-day dosing and consecutive-day dosing, respectively. There were no statistical differences in pretreatment Hb (8.15 vs. 8.67 g/dL, P = 0.288) and sF (5.42 vs. 7.29 µg/L, P = 0.175) between alternate-day dosing and consecutive-day dosing, respectively. At D30, the proportions of patients with ≥ 2 g/dL Hb increase were 63.2% vs 61.1% (absolute difference 2.1%; 95% confidence interval [95%CI] -28.7% to 3.3%, P = 0.14 for non-inferiority), while the proportions of patients with ≥ 1 g/dL Hb increase were 100% vs 83.3% (absolute difference 16.7%; 95%CI -0.16% to 38.6%, P = 0.002, for non-inferiority) in alternate-day dosing and consecutive-day dosing, respectively. There were no statistical differences in Hb (D30; 10.35 vs. 11.12 g/dL, P = 0.135, and D90; 12.02 vs. 12.32 g/dL, P = 0.603) and sF (D30; 39.36 vs. 47.33 µg/L, P = 0.349, and D90; 38.53 vs. 48.70 µg/L, P = 0.349) between alternate-day dosing and consecutive-day dosing at D30 and D90 post-treatment, respectively. Gastrointestinal disturbances were reported of 26.3% in alternate-day dosing vs. of 31.3% in consecutive-day dosing, P = 0.748)
Conclusion
Alternate-day single dosing of iron supplements may be an alternative treatment for IDA, especially in patients who do not require a rapid correction of anemia. This trial is actively enrolling.
Keyword(s): Female, Ferritin, Hepcidin, Iron deficiency anemia
Abstract: EP845
Type: E-Poster Presentation
Session title: Iron metabolism, deficiency and overload
Background
The consecutive-day split dosing of iron supplements is the most common treatment of iron deficiency anemia (IDA) in clinical practice. Although recent ferrokinetic studies in iron-deficient young women demonstrated greater iron absorption with alternate-day single dosing compared to conventional twice-daily split dosing, there have been no studies evaluating long-term efficacy between these two schemes in treatment of IDA in reproductive-aged women.
Aims
This open-label, non-inferiority, randomized controlled trial (TCTR20200614001) primarily aims to evaluate efficacy and safety between alternate-day single dosing of iron supplements versus consecutive-day split dosing in reproductive-aged women with IDA.
Methods
Women aged 18-50 years were eligible for enrollment. IDA was defined by serum ferritin levels (sF) <30 µg/L. Patients randomized in alternate-day dosing were given single doses of ferrous sulfate 400 mg on alternate days, while patients in consecutive-day dosing were given ferrous sulfate 200 mg twice daily. The primary outcome was the proportion of patients with ≥ 2 g/dL hemoglobin (Hb) increase at day 30 (D30) post-treatment. The secondary outcomes were the proportion of patients with ≥ 1 g Hb increase at D30; Hb and sF at D30 and D90; and incidence of adverse events.
Results
Of 37 in preliminary analysis, 19 and 18 were randomized to alternate-day dosing and consecutive-day dosing, respectively. There were no statistical differences in pretreatment Hb (8.15 vs. 8.67 g/dL, P = 0.288) and sF (5.42 vs. 7.29 µg/L, P = 0.175) between alternate-day dosing and consecutive-day dosing, respectively. At D30, the proportions of patients with ≥ 2 g/dL Hb increase were 63.2% vs 61.1% (absolute difference 2.1%; 95% confidence interval [95%CI] -28.7% to 3.3%, P = 0.14 for non-inferiority), while the proportions of patients with ≥ 1 g/dL Hb increase were 100% vs 83.3% (absolute difference 16.7%; 95%CI -0.16% to 38.6%, P = 0.002, for non-inferiority) in alternate-day dosing and consecutive-day dosing, respectively. There were no statistical differences in Hb (D30; 10.35 vs. 11.12 g/dL, P = 0.135, and D90; 12.02 vs. 12.32 g/dL, P = 0.603) and sF (D30; 39.36 vs. 47.33 µg/L, P = 0.349, and D90; 38.53 vs. 48.70 µg/L, P = 0.349) between alternate-day dosing and consecutive-day dosing at D30 and D90 post-treatment, respectively. Gastrointestinal disturbances were reported of 26.3% in alternate-day dosing vs. of 31.3% in consecutive-day dosing, P = 0.748)

Conclusion
Alternate-day single dosing of iron supplements may be an alternative treatment for IDA, especially in patients who do not require a rapid correction of anemia. This trial is actively enrolling.
Keyword(s): Female, Ferritin, Hepcidin, Iron deficiency anemia


