
Contributions
Abstract: EP844
Type: E-Poster Presentation
Session title: Iron metabolism, deficiency and overload
Background
Hematopoietic cell transplantation (HCT) is associated with important changes in erythropoiesis and alterations of iron metabolism. Production of hepcidin, the regulator of iron metabolism, is increased in iron overload or inflammation and decreased with iron deficiency or activated erythropoiesis. Erythroferrone (ERFE) has been recently identified as the erythroid regulator of hepcidin.
Aims
We investigated erythropoiesis and iron metabolism after before and up to 180 days after allogeneic HCT in 70 patients randomized between erythropoietin (EPO) treatment or no EPO. We investigated serially serum levels of CRP (marker of inflammation); soluble transferrin receptor (sTfR, quantitative measure of erythropoietic activity); serum iron and transferrin saturation (Tsat; iron availability for erythropoiesis) and ferritin (iron stores); hepcidin (regulator of iron metabolism); and ERFE (erythroid regulator of hepcidin). We also sought to identify biological and clinical factors associated with serum hepcidin and ERFE levels.
Methods
Serum hepcidin was measured by a liquid chromatography/tandem mass spectrometry method and serum erythroferrone by a specific ELISA kit (Intrinsic Lifesciences, The Bio Iron Company, USA), while the other parameters were measured by routine methods. A generalized linear mixed model (GLMM) was used to compare their evolution with respect to time and treatment arm. Univariate and multivariate linear regression models were used to identify biological and clinical factors (age, sex, diagnosis, disease risk index, HCT-CI, conditioning, donor type, graft type, ABO, red blood cell and platelet transfusions, CMV status, Graft-versus-Host Disease, infections and EPO treatment) associated with hepcidin and serum ERFE levels at different times after transplantation.
Results
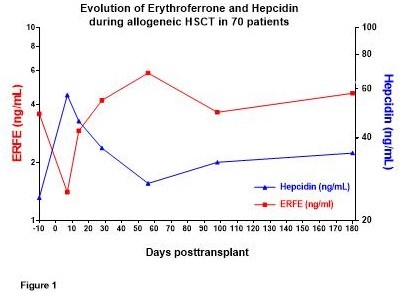
Serum ERFE correlated overall with sTfR (p<0.0001), reticulocytes (p<0.0001) and inversely with hepcidin (p=0.0095). Serum ERFE paralleled sTfR levels, with a drop during conditioning followed by recovery during engraftment. Inversely, hepcidin peaked during conditioning and decreased during engraftment (Figure 1). ERFE and hepcidin levels were not significantly different between the control and EPO arms. Multivariate analyses showed that during conditioning and engraftment and at day 100 (R²=0.17 to 0.61, p<0.001), the major determinant of ERFE was erythropoietic activity (sTfR or reticulocytes or serum Epo). Pretransplant hepcidin was associated (R²=0.52, p<0.0001) with previous RBC transfusions (p=0.02) and serum ferritin (p=0.001). In the course of transplantation (day 0 to day 180), the major determinants of hepcidin levels (R² 0.35 to 0.60, p<0.0001) were iron status (ferritin at all time points (all p<0.0001) and Tsat at day 56 (p=0.0003)) and erythropoietic activity (sTfR or reticulocytes or ERFE (p ranging from 0.05 to 0.0001)), while inflammation and clinical parameters had no detectable influence. Indeed, hepcidin levels remained significantly higher in patients with high compared to low pretransplant ferritin (p<0.0001).
Conclusion
The evolution of ERFE, hepcidin and other parameters of iron status and erythropoietic activity after allogeneic HCT with or without EPO therapy confirms the strong impact of ERFE on hepcidin production and supports the erythropoietic origin of ERFE.
Keyword(s): Allogeneic stem cell transplant, Erythropoieisis, Hepcidin, Iron metabolism
Abstract: EP844
Type: E-Poster Presentation
Session title: Iron metabolism, deficiency and overload
Background
Hematopoietic cell transplantation (HCT) is associated with important changes in erythropoiesis and alterations of iron metabolism. Production of hepcidin, the regulator of iron metabolism, is increased in iron overload or inflammation and decreased with iron deficiency or activated erythropoiesis. Erythroferrone (ERFE) has been recently identified as the erythroid regulator of hepcidin.
Aims
We investigated erythropoiesis and iron metabolism after before and up to 180 days after allogeneic HCT in 70 patients randomized between erythropoietin (EPO) treatment or no EPO. We investigated serially serum levels of CRP (marker of inflammation); soluble transferrin receptor (sTfR, quantitative measure of erythropoietic activity); serum iron and transferrin saturation (Tsat; iron availability for erythropoiesis) and ferritin (iron stores); hepcidin (regulator of iron metabolism); and ERFE (erythroid regulator of hepcidin). We also sought to identify biological and clinical factors associated with serum hepcidin and ERFE levels.
Methods
Serum hepcidin was measured by a liquid chromatography/tandem mass spectrometry method and serum erythroferrone by a specific ELISA kit (Intrinsic Lifesciences, The Bio Iron Company, USA), while the other parameters were measured by routine methods. A generalized linear mixed model (GLMM) was used to compare their evolution with respect to time and treatment arm. Univariate and multivariate linear regression models were used to identify biological and clinical factors (age, sex, diagnosis, disease risk index, HCT-CI, conditioning, donor type, graft type, ABO, red blood cell and platelet transfusions, CMV status, Graft-versus-Host Disease, infections and EPO treatment) associated with hepcidin and serum ERFE levels at different times after transplantation.
Results
Serum ERFE correlated overall with sTfR (p<0.0001), reticulocytes (p<0.0001) and inversely with hepcidin (p=0.0095). Serum ERFE paralleled sTfR levels, with a drop during conditioning followed by recovery during engraftment. Inversely, hepcidin peaked during conditioning and decreased during engraftment (Figure 1). ERFE and hepcidin levels were not significantly different between the control and EPO arms. Multivariate analyses showed that during conditioning and engraftment and at day 100 (R²=0.17 to 0.61, p<0.001), the major determinant of ERFE was erythropoietic activity (sTfR or reticulocytes or serum Epo). Pretransplant hepcidin was associated (R²=0.52, p<0.0001) with previous RBC transfusions (p=0.02) and serum ferritin (p=0.001). In the course of transplantation (day 0 to day 180), the major determinants of hepcidin levels (R² 0.35 to 0.60, p<0.0001) were iron status (ferritin at all time points (all p<0.0001) and Tsat at day 56 (p=0.0003)) and erythropoietic activity (sTfR or reticulocytes or ERFE (p ranging from 0.05 to 0.0001)), while inflammation and clinical parameters had no detectable influence. Indeed, hepcidin levels remained significantly higher in patients with high compared to low pretransplant ferritin (p<0.0001).

Conclusion
The evolution of ERFE, hepcidin and other parameters of iron status and erythropoietic activity after allogeneic HCT with or without EPO therapy confirms the strong impact of ERFE on hepcidin production and supports the erythropoietic origin of ERFE.
Keyword(s): Allogeneic stem cell transplant, Erythropoieisis, Hepcidin, Iron metabolism


