
Contributions
Abstract: EP813
Type: E-Poster Presentation
Session title: Infections in hematology (incl. supportive care/therapy)
Background
An aberrant increase of erythroid progenitors in circulation have been reported in severe COVID-19 cases and correlates with severity and mortality. The infectivity of SARS-CoV-2 in HSPCs and more particularly in erythroid progenitors have not been characterized. In this report, we have studied HSPCs and different erythroid progenitor populations to assess if they can be infected by SARS-CoV-2.
Aims
Demonstrate that SARS-CoV-2 can infect erythroid progenitors and disrupt erythropoiesis causing the aberrant increase of nucleated red blood cells observed in severe COVID-19 patients.
Methods
Clinical data: A sample of 30 patients who were being treated for COVID-19 on intensive care units at King’s College Hospital; RNAseq data import and analysis of ACE2 expression: RNAseq dataset was downloaded from GSE118537; Isolation of mononuclear cells from human cord blood, bone marrow and peripheral blood; Flow cytometry analysis and cell sorting; mRNA quantification by RT-qPCR; Immunostaining, confocal microscopy and immunofluorescence quantification of ACE2 and TMPRSS2; SARS-CoV-2 production and infection; Colony-forming Unit (CFU) assay
Results
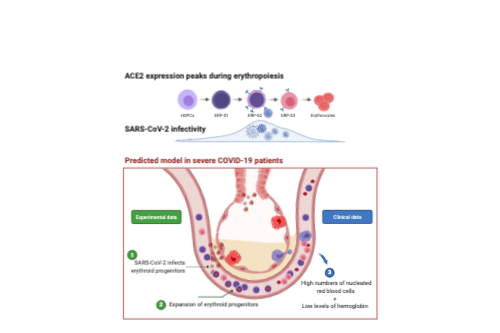
- Patients treated for severe COVID-19 show a rapid and profound decline in hemoglobin following admission. Interestingly, this is associated with the appearance of circulating nucleated red cells.
- Publicly available datasets indicate that ACE2 and TMPRSS2 expression is scarce among hematopoietic cell types, and absent on human HSPCs. Progenitors of the erythroid lineage appear to be the only cell types expressing both ACE2 and TMPRSS2 among the cells present in the bone marrow (The Human Cell Atlas Bone Marrow Single-Cell Interactive Web Portal). Intrigued by this observation, we used detailed datasets of in vitro human erythropoiesis and we found that ACE2 expression peaks during erythropoiesis. Erythroid progenitors of Stage 1 (ERP-S1) can be defined as CD34+CD117+CD71+CD235a-, ERP-S2 as CD34-CD117+CD71+CD235a- and ERP-S3 as CD34-CD117-CD71+CD235a+.
- ERP-S2 cells are highly susceptible to SARS-CoV-2 infection. Importantly, our results showed that SARS-CoV-2 can infect and amplify its genome in erythroid progenitors of Stage 2 and Stage 3 but is not able to bind and infect HSPCs from the bone marrow.
- We also infected the erythroid progenitors from peripheral blood at lower MOI and we obtained similar results. After being in the presence of SARS-CoV-2, erythroid progenitors produce more colonies. We detected the virus in the colonies from ERP-S2, indicating that SARS-CoV-2 remains in ERP-S2 after 14 days of the initial infection.
Conclusion
We document here that intensive care COVID19 patients suffer a profound decline in hemoglobin levels but show an increase of circulating nucleated red cells, suggesting that SARS-CoV-2 infection either directly or indirectly induces stress erythropoiesis. Early erythroid progenitors, defined as CD34-CD117+CD71+CD235a-, show the highest levels of ACE2 and constitute the primary target cell to be infected during erythropoiesis. SARS-CoV-2 causes the expansion of colony formation by erythroid progenitors and can be detected in these cells after two weeks of the initial infection. Our findings constitute the first report of SARS-CoV-2 infectivity in erythroid progenitor cells and can contribute to understanding both the clinical symptoms of severe COVID19 patients and how the virus can spread through the circulation to produce local inflammation in tissues, including the bone marrow.
Keyword(s): COVID-19, Erythroid progenitor, Infection
Abstract: EP813
Type: E-Poster Presentation
Session title: Infections in hematology (incl. supportive care/therapy)
Background
An aberrant increase of erythroid progenitors in circulation have been reported in severe COVID-19 cases and correlates with severity and mortality. The infectivity of SARS-CoV-2 in HSPCs and more particularly in erythroid progenitors have not been characterized. In this report, we have studied HSPCs and different erythroid progenitor populations to assess if they can be infected by SARS-CoV-2.
Aims
Demonstrate that SARS-CoV-2 can infect erythroid progenitors and disrupt erythropoiesis causing the aberrant increase of nucleated red blood cells observed in severe COVID-19 patients.
Methods
Clinical data: A sample of 30 patients who were being treated for COVID-19 on intensive care units at King’s College Hospital; RNAseq data import and analysis of ACE2 expression: RNAseq dataset was downloaded from GSE118537; Isolation of mononuclear cells from human cord blood, bone marrow and peripheral blood; Flow cytometry analysis and cell sorting; mRNA quantification by RT-qPCR; Immunostaining, confocal microscopy and immunofluorescence quantification of ACE2 and TMPRSS2; SARS-CoV-2 production and infection; Colony-forming Unit (CFU) assay
Results
- Patients treated for severe COVID-19 show a rapid and profound decline in hemoglobin following admission. Interestingly, this is associated with the appearance of circulating nucleated red cells.
- Publicly available datasets indicate that ACE2 and TMPRSS2 expression is scarce among hematopoietic cell types, and absent on human HSPCs. Progenitors of the erythroid lineage appear to be the only cell types expressing both ACE2 and TMPRSS2 among the cells present in the bone marrow (The Human Cell Atlas Bone Marrow Single-Cell Interactive Web Portal). Intrigued by this observation, we used detailed datasets of in vitro human erythropoiesis and we found that ACE2 expression peaks during erythropoiesis. Erythroid progenitors of Stage 1 (ERP-S1) can be defined as CD34+CD117+CD71+CD235a-, ERP-S2 as CD34-CD117+CD71+CD235a- and ERP-S3 as CD34-CD117-CD71+CD235a+.
- ERP-S2 cells are highly susceptible to SARS-CoV-2 infection. Importantly, our results showed that SARS-CoV-2 can infect and amplify its genome in erythroid progenitors of Stage 2 and Stage 3 but is not able to bind and infect HSPCs from the bone marrow.
- We also infected the erythroid progenitors from peripheral blood at lower MOI and we obtained similar results. After being in the presence of SARS-CoV-2, erythroid progenitors produce more colonies. We detected the virus in the colonies from ERP-S2, indicating that SARS-CoV-2 remains in ERP-S2 after 14 days of the initial infection.

Conclusion
We document here that intensive care COVID19 patients suffer a profound decline in hemoglobin levels but show an increase of circulating nucleated red cells, suggesting that SARS-CoV-2 infection either directly or indirectly induces stress erythropoiesis. Early erythroid progenitors, defined as CD34-CD117+CD71+CD235a-, show the highest levels of ACE2 and constitute the primary target cell to be infected during erythropoiesis. SARS-CoV-2 causes the expansion of colony formation by erythroid progenitors and can be detected in these cells after two weeks of the initial infection. Our findings constitute the first report of SARS-CoV-2 infectivity in erythroid progenitor cells and can contribute to understanding both the clinical symptoms of severe COVID19 patients and how the virus can spread through the circulation to produce local inflammation in tissues, including the bone marrow.
Keyword(s): COVID-19, Erythroid progenitor, Infection


