
Contributions
Abstract: EP807
Type: E-Poster Presentation
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Obinutuzumab (G)-chemotherapy (chemo) has demonstrated improved progression-free survival compared with rituximab (R)-chemo in previously untreated advanced follicular lymphoma (FL). G is currently administered by intravenous infusion over ~3–4 hours. A shorter duration of infusion in Cycle (C) 2 and subsequent cycles, as is standard practice with R, could improve convenience for patients (pts) and efficiency for infusion facilities.
Aims
We report the primary analysis of the prospective, open-label, multicenter, single-arm, Phase IV, GAZELLE study (NCT03817853), which evaluated the safety of G administered as a 90-minute (min) SDI from C2 onwards in pts with FL.
Methods
Pts with previously untreated FL who met Groupe d'Etude des Lymphomes Folliculaires (GELF) criteria received G (1000mg) intravenously on Day (D) 1, 8 and 15 of C1, and on D1 thereafter, plus chemo (bendamustine, CHOP or CVP) for 6–8 cycles. In C1, pts received G at the standard infusion rate. Pts without a Grade (Gr) ≥3 infusion-related reaction (IRR) in C1 were eligible to receive G as a 90-min SDI from C2. Pts with a Gr 3 IRR in C1 received the standard G infusion in C2, and were eligible for G SDI in subsequent cycles if no Gr ≥3 IRRs occurred. Pts with a second Gr 3/4 IRR discontinued G. At the end of induction (EOI), responding pts received maintenance G (1000mg) as SDI for 2 years or until disease progression (PD). The primary endpoint was incidence of Gr ≥3 IRRs during C2. IRRs were defined as any event occurring ≤24 hours from infusion judged to be related to treatment. Secondary endpoints included adverse events (AEs) and investigator-assessed overall response rate at EOI.
Results
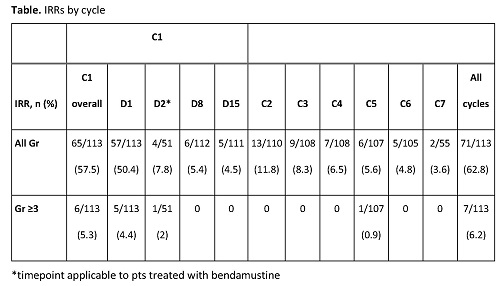
As of December 3, 2020, 113 pts had received study treatment. Median age was 62.0 years, 50.4% were male, 61.9% had stage IV FL and 45.1% were classified as high-risk FLIPI. Of the 110 pts who were eligible for G SDI from C2, no pt experienced a Gr ≥3 IRR with SDI in C2 (Table). One pt experienced a Gr 3 IRR with SDI in C5, presenting hypertension. All other IRRs with SDI were Gr 1/2. No Gr 4/5 IRRs were reported. Other AEs were similar to those observed in previous studies. At the clinical cut-off date, 104 pts had a CT imaging-based response assessment at EOI and 9 pts had no response assessment; 76/113 (67.3%) pts had a complete response, 22 (19.5%) had a partial response, and six pts (5.8%) had PD.
Conclusion
In GAZELLE, G SDI in C2 and beyond appeared to be safe. No Gr 3 IRRs were observed in C2 and only one Gr 3 IRR was reported in subsequent cycles. No new safety signals were reported and 67.3% of pts achieved a CR. These data suggest that the safety and efficacy profile of G SDI is comparable with the established profile of G in advanced FL.
Keyword(s): Adverse reaction, Follicular lymphoma, Obinutuzumab
Abstract: EP807
Type: E-Poster Presentation
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Obinutuzumab (G)-chemotherapy (chemo) has demonstrated improved progression-free survival compared with rituximab (R)-chemo in previously untreated advanced follicular lymphoma (FL). G is currently administered by intravenous infusion over ~3–4 hours. A shorter duration of infusion in Cycle (C) 2 and subsequent cycles, as is standard practice with R, could improve convenience for patients (pts) and efficiency for infusion facilities.
Aims
We report the primary analysis of the prospective, open-label, multicenter, single-arm, Phase IV, GAZELLE study (NCT03817853), which evaluated the safety of G administered as a 90-minute (min) SDI from C2 onwards in pts with FL.
Methods
Pts with previously untreated FL who met Groupe d'Etude des Lymphomes Folliculaires (GELF) criteria received G (1000mg) intravenously on Day (D) 1, 8 and 15 of C1, and on D1 thereafter, plus chemo (bendamustine, CHOP or CVP) for 6–8 cycles. In C1, pts received G at the standard infusion rate. Pts without a Grade (Gr) ≥3 infusion-related reaction (IRR) in C1 were eligible to receive G as a 90-min SDI from C2. Pts with a Gr 3 IRR in C1 received the standard G infusion in C2, and were eligible for G SDI in subsequent cycles if no Gr ≥3 IRRs occurred. Pts with a second Gr 3/4 IRR discontinued G. At the end of induction (EOI), responding pts received maintenance G (1000mg) as SDI for 2 years or until disease progression (PD). The primary endpoint was incidence of Gr ≥3 IRRs during C2. IRRs were defined as any event occurring ≤24 hours from infusion judged to be related to treatment. Secondary endpoints included adverse events (AEs) and investigator-assessed overall response rate at EOI.
Results
As of December 3, 2020, 113 pts had received study treatment. Median age was 62.0 years, 50.4% were male, 61.9% had stage IV FL and 45.1% were classified as high-risk FLIPI. Of the 110 pts who were eligible for G SDI from C2, no pt experienced a Gr ≥3 IRR with SDI in C2 (Table). One pt experienced a Gr 3 IRR with SDI in C5, presenting hypertension. All other IRRs with SDI were Gr 1/2. No Gr 4/5 IRRs were reported. Other AEs were similar to those observed in previous studies. At the clinical cut-off date, 104 pts had a CT imaging-based response assessment at EOI and 9 pts had no response assessment; 76/113 (67.3%) pts had a complete response, 22 (19.5%) had a partial response, and six pts (5.8%) had PD.

Conclusion
In GAZELLE, G SDI in C2 and beyond appeared to be safe. No Gr 3 IRRs were observed in C2 and only one Gr 3 IRR was reported in subsequent cycles. No new safety signals were reported and 67.3% of pts achieved a CR. These data suggest that the safety and efficacy profile of G SDI is comparable with the established profile of G in advanced FL.
Keyword(s): Adverse reaction, Follicular lymphoma, Obinutuzumab


