
Contributions
Abstract: EP806
Type: E-Poster Presentation
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Despite recent improvements in chemo-immunotherapy and novel therapies for mantle cell lymphoma (MCL), patients continue to experience heterogeneous clinical outcomes. Validated predictors of high-risk for adverse outcome include high-risk MCL International Prognostic Index (MIPI), Ki-67 ≥30% and blastic or pleomorphic histological variants. MCL harbouring pathogenic TP53 mutations treated on Nordic MCL2/MCL3 clinical trials have an inferior outcome, therefore detecting these mutations prior to treatment initiation may alter the treatment pathway utilising targeted therapy or a clinical trial. Missense TP53 mutations lead to accumulation of p53 within the cell, which is detectable by immunohistochemistry, whereas clinically relevant deletions or other TP53 mutations are only detected by Next Generation Sequencing (NGS).
Aims
We aimed to evaluate (1) the concordance of p53 immunohistochemistry and TP53 NGS in detecting high-risk missense TP53 mutations, (2) incidence of non-missense TP53 disruption in diagnostic specimens of MCL patients treated on the Nordic MCL2 protocol.
Methods
We identified patients treated between 2007-2019 on a modified Nordic MCL2 protocol (alternating Rituximab-CHOP/Rituximab-Cytarabine induction followed by BEAM-conditioned autologous stem cell transplantation). All patients undergoing HD-ASCT achieved VGPR/CR with induction therapy, as determined by Lugano criteria and were MRD negative. p53 expression by immunohistochemistry in diagnostic specimens was compared with NGS of TP53 and correlated with clinical outcomes. Tumour sections with ≥30% of neoplastic cells staining positive for TP53 by IHC were defined as p53 positive as previously described. NGS called pathogenic TP53 mutations occurring at ≥5% VAF, with pathogenicity inferred from well-curated databases. All data is calculated from date of commencing Nordic protocol to date of censoring (death or end of study).
Results
42 patients were treated with MCL2 between 2007-2019, of whom 38 had either DNA, tumour block or both available for TP53 mutation analysis. 34 (81%) patients were male and the median age was 59 years (range 37-67). 16 (41%) of patients had high-risk MIPI and 4 (9.5%) had non-classical MCL subtypes. Median overall survival (OS) was not reached and disease-free survival was 6.5 years. There was no transplant-related mortality. High-risk MIPI (p=0.078), Ki67≥30% (p=0.50), SOX11<10% (p=0.06) and non-classical MCL subtype (p=0.56) were not associated with inferior OS, likely due to the small cohort size.
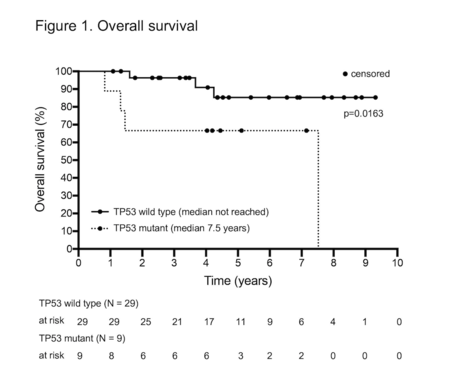
Pathogenic TP53 mutations by NGS (6 cases) or p53 expression by IHC (4 cases) were detected in 9 patients (24%) and associated with inferior OS (p=0.0163) (Figure 1). Paired p53 IHC was unavailable for 5/6 TP53 mutated cases by NGS, however P53 was expressed in the remaining case. One of 4 P53 IHC positive cases lacked a corresponding NGS sample. Two P53 positive cases occurred in patients with NGS detectable mutations not classified as pathogenic: these patients remain in CR1 at 38 and 55 months from HD-ASCT respectively. There was 100% concordance between paired TP53 wild type cases and TP53 <30% by IHC (20 cases).
Conclusion
The presence of pathogenic TP53 was associated with adverse overall survival in this cohort. P53 IHC flagged two cases with TP53 mutations of uncertain significance by NGS. The implications of this finding must be explored in larger patient series, but suggest that IHC may not detect pathogenic TP53 mutations with sufficient accuracy to direct treatment pathways. All detected TP53 mutations were missense, reflecting the small study cohort.
Keyword(s): Autologous bone marrow transplant, Immunohistochemistry, Mantle cell lymphoma, P53
Abstract: EP806
Type: E-Poster Presentation
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Despite recent improvements in chemo-immunotherapy and novel therapies for mantle cell lymphoma (MCL), patients continue to experience heterogeneous clinical outcomes. Validated predictors of high-risk for adverse outcome include high-risk MCL International Prognostic Index (MIPI), Ki-67 ≥30% and blastic or pleomorphic histological variants. MCL harbouring pathogenic TP53 mutations treated on Nordic MCL2/MCL3 clinical trials have an inferior outcome, therefore detecting these mutations prior to treatment initiation may alter the treatment pathway utilising targeted therapy or a clinical trial. Missense TP53 mutations lead to accumulation of p53 within the cell, which is detectable by immunohistochemistry, whereas clinically relevant deletions or other TP53 mutations are only detected by Next Generation Sequencing (NGS).
Aims
We aimed to evaluate (1) the concordance of p53 immunohistochemistry and TP53 NGS in detecting high-risk missense TP53 mutations, (2) incidence of non-missense TP53 disruption in diagnostic specimens of MCL patients treated on the Nordic MCL2 protocol.
Methods
We identified patients treated between 2007-2019 on a modified Nordic MCL2 protocol (alternating Rituximab-CHOP/Rituximab-Cytarabine induction followed by BEAM-conditioned autologous stem cell transplantation). All patients undergoing HD-ASCT achieved VGPR/CR with induction therapy, as determined by Lugano criteria and were MRD negative. p53 expression by immunohistochemistry in diagnostic specimens was compared with NGS of TP53 and correlated with clinical outcomes. Tumour sections with ≥30% of neoplastic cells staining positive for TP53 by IHC were defined as p53 positive as previously described. NGS called pathogenic TP53 mutations occurring at ≥5% VAF, with pathogenicity inferred from well-curated databases. All data is calculated from date of commencing Nordic protocol to date of censoring (death or end of study).
Results
42 patients were treated with MCL2 between 2007-2019, of whom 38 had either DNA, tumour block or both available for TP53 mutation analysis. 34 (81%) patients were male and the median age was 59 years (range 37-67). 16 (41%) of patients had high-risk MIPI and 4 (9.5%) had non-classical MCL subtypes. Median overall survival (OS) was not reached and disease-free survival was 6.5 years. There was no transplant-related mortality. High-risk MIPI (p=0.078), Ki67≥30% (p=0.50), SOX11<10% (p=0.06) and non-classical MCL subtype (p=0.56) were not associated with inferior OS, likely due to the small cohort size.
Pathogenic TP53 mutations by NGS (6 cases) or p53 expression by IHC (4 cases) were detected in 9 patients (24%) and associated with inferior OS (p=0.0163) (Figure 1). Paired p53 IHC was unavailable for 5/6 TP53 mutated cases by NGS, however P53 was expressed in the remaining case. One of 4 P53 IHC positive cases lacked a corresponding NGS sample. Two P53 positive cases occurred in patients with NGS detectable mutations not classified as pathogenic: these patients remain in CR1 at 38 and 55 months from HD-ASCT respectively. There was 100% concordance between paired TP53 wild type cases and TP53 <30% by IHC (20 cases).

Conclusion
The presence of pathogenic TP53 was associated with adverse overall survival in this cohort. P53 IHC flagged two cases with TP53 mutations of uncertain significance by NGS. The implications of this finding must be explored in larger patient series, but suggest that IHC may not detect pathogenic TP53 mutations with sufficient accuracy to direct treatment pathways. All detected TP53 mutations were missense, reflecting the small study cohort.
Keyword(s): Autologous bone marrow transplant, Immunohistochemistry, Mantle cell lymphoma, P53


