
Contributions
Abstract: EP805
Type: E-Poster Presentation
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
There is a lack of randomized trials directly comparing zanubrutinib with chemoimmunotherapy in Waldenström Macroglobulinemia (WM).
Aims
This study aimed to indirectly compare zanubrutinib with bendamustine-rituximab (BR) and with dexamethasone-rituximab-cyclophosphamide (DRC) separately through matching-adjusted indirect comparisons (MAIC).
Methods
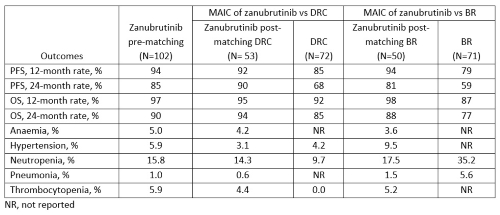
MAIC were conducted to re-weight the individual data of 102 WM patients (83 relapsed/refractory [R/R] and 19 treatment-naïve [TN]) treated with zanubrutinib in the ASPEN trial (NCT03053440) so that the weighted average baseline characteristics of patients treated with zanubrutinib matched those of 71 R/R patients treated with BR, and 72 TN patients treated with DRC separately. Matching variables for MAIC with BR included age, prior lines of therapy, IgM concentration, International Prognostic Scoring System for WM score, and extramedullary disease (EMD); and for MAIC with DRC included age, platelet count, hemoglobin concentration, and EMD. Kaplan-Meier curves of progression-free survival (PFS) and overall survival (OS) of comparators were digitized to re-create patient-level data. Comparisons of survival and adverse event incidence between treatments were conducted using Cox proportional hazards models and modified Poisson models.
Results
Compared to DRC, zanubrutinib was associated with significantly longer PFS (hazard ratio [HR]: 0.39 [95% confidence interval 0.18-0.82] and 0.35 [0.14-0.86] pre- and post-matching, respectively) and insignificantly longer OS (HR: 0.56 [0.20-1.53] and 0.47 [0.14-1.62] pre- and post-matching, respectively), and insignificantly higher incidences of neutropenia (risk ratio [RR]: 1.63 [0.71-3.77] and 1.47 [0.58-3.74] pre- and post-matching, respectively). Compared to BR, zanubrutinib was associated with significantly longer PFS (HR: 0.32 [0.15-0.69] and 0.37 [0.15-0.91] pre- and post-matching, respectively), significantly longer OS (HR: 0.31 [0.12, 0.80] and 0.29 [0.10-0.85] pre- and post-matching, respectively), significantly lower incidences of neutropenia (RR: 0.45 [0.26-0.78] and 0.50 [0.27-0.91] pre- and post-matching, respectively) and insignificantly lower incidences of pneumonia (RR: 0.18 [0.02-1.55] and 0.26 [0.03-2.28] pre- and post-matching, respectively).
Conclusion
Zanubrutinib demonstrated longer PFS than DRC, longer PFS and OS than BR in WM, and lower incidence of neutropenia before and after matching adjustment based on patient characteristics.
Keyword(s): Rituximab, Waldenstrom's macroglobulinemia
Abstract: EP805
Type: E-Poster Presentation
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
There is a lack of randomized trials directly comparing zanubrutinib with chemoimmunotherapy in Waldenström Macroglobulinemia (WM).
Aims
This study aimed to indirectly compare zanubrutinib with bendamustine-rituximab (BR) and with dexamethasone-rituximab-cyclophosphamide (DRC) separately through matching-adjusted indirect comparisons (MAIC).
Methods
MAIC were conducted to re-weight the individual data of 102 WM patients (83 relapsed/refractory [R/R] and 19 treatment-naïve [TN]) treated with zanubrutinib in the ASPEN trial (NCT03053440) so that the weighted average baseline characteristics of patients treated with zanubrutinib matched those of 71 R/R patients treated with BR, and 72 TN patients treated with DRC separately. Matching variables for MAIC with BR included age, prior lines of therapy, IgM concentration, International Prognostic Scoring System for WM score, and extramedullary disease (EMD); and for MAIC with DRC included age, platelet count, hemoglobin concentration, and EMD. Kaplan-Meier curves of progression-free survival (PFS) and overall survival (OS) of comparators were digitized to re-create patient-level data. Comparisons of survival and adverse event incidence between treatments were conducted using Cox proportional hazards models and modified Poisson models.
Results
Compared to DRC, zanubrutinib was associated with significantly longer PFS (hazard ratio [HR]: 0.39 [95% confidence interval 0.18-0.82] and 0.35 [0.14-0.86] pre- and post-matching, respectively) and insignificantly longer OS (HR: 0.56 [0.20-1.53] and 0.47 [0.14-1.62] pre- and post-matching, respectively), and insignificantly higher incidences of neutropenia (risk ratio [RR]: 1.63 [0.71-3.77] and 1.47 [0.58-3.74] pre- and post-matching, respectively). Compared to BR, zanubrutinib was associated with significantly longer PFS (HR: 0.32 [0.15-0.69] and 0.37 [0.15-0.91] pre- and post-matching, respectively), significantly longer OS (HR: 0.31 [0.12, 0.80] and 0.29 [0.10-0.85] pre- and post-matching, respectively), significantly lower incidences of neutropenia (RR: 0.45 [0.26-0.78] and 0.50 [0.27-0.91] pre- and post-matching, respectively) and insignificantly lower incidences of pneumonia (RR: 0.18 [0.02-1.55] and 0.26 [0.03-2.28] pre- and post-matching, respectively).

Conclusion
Zanubrutinib demonstrated longer PFS than DRC, longer PFS and OS than BR in WM, and lower incidence of neutropenia before and after matching adjustment based on patient characteristics.
Keyword(s): Rituximab, Waldenstrom's macroglobulinemia


