
Contributions
Abstract: EP796
Type: E-Poster Presentation
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
LPL/WM is a rare lymphoproliferative malignancy. Although therapeutic advances have been made in LPL/WM over the past decades, it is unclear how these advances have impacted the population-level survival of LPL/WM patients, especially among contemporary diagnosed patients. This inconclusiveness relates to the lack of population-based studies in LPL/WM.
Aims
This nationwide, population-based study aimed to assess trends in first-line therapy and relative survival (RS) among LPL/WM patients diagnosed in the Netherlands during a 30-year period.
Methods
All LPL/WM patients diagnosed between 1989-2018—with survival follow-up through 2020—were selected from the Netherlands Cancer Registry (NCR). Data on primary therapy—that is, no anti-neoplastic therapy (i.e. watch-and-wait) and anti-neoplastic therapy (e.g. chemotherapy)—were available in the NCR. Information on the use of rituximab was available for patients diagnosed as from 2007. Patients were categorized into four periods (1989-1995, 1996-2002, 2003-2010 and 2011-2018), and three age groups (<65 (n=383), 66-75 (n=384) and >75 years (n=386)). Data on the exact anti-neoplastic therapy, asymptomatic/symptomatic disease, and IPSS risk group were available for patients diagnosed as from 2014. The exact anti-neoplastic regimens were presented for patients with symptomatic disease, stratified by year of diagnosis, the three abovementioned age groups, and IPSS risk group (i.e. low, intermediate, and high). RS was calculated to estimate disease-specific survival.
Results
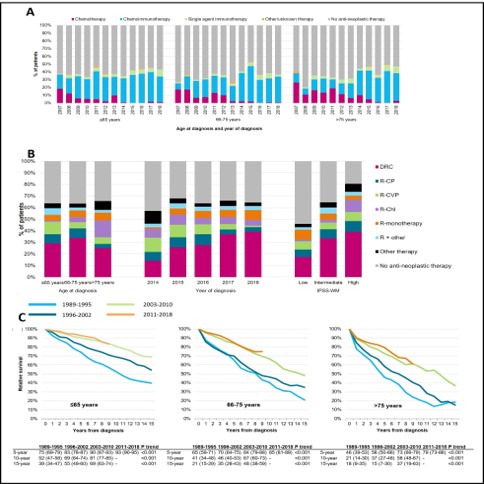
A total of 6,232 LPL/WM patients (median age 70 years; 61% males) were included in the study. Due to the broader application of an initial watch-and-wait approach, treatment with anti-neoplastic agents at diagnosis gradually decreased over time, irrespective of age. The proportion of patients receiving upfront anti-neoplastic therapy was 41%, 38%, and 40% across the three age groups during 2011-2018. Data from 2007 onwards showed that the use of chemotherapy alone decreased over time, following an increased application of chemoimmunotherapy (Figure 1A). Symptomatic disease was present in 60% of patients diagnosed during 2014-2018. Detailed data on primary therapy among 960 symptomatic patients revealed that DRC was the most frequently applied therapeutic regimen across all age groups, of which its use increased from 14% to 39% between 2014 and 2018. Consequently, the use of R-C(V)P decreased between 2014 and 2018. The use of anti-neoplastic therapy increased with advancing IPSS risk group (Figure 1B). Five-year RS increased significantly across all age groups between the first and last calendar period. Specifically, 5-year RS (95% confidence intervals) was 75% (69%>79%), 65% (59%>71%), and 46% (39%>53%) in 1989-1995 across the three age groups, compared with 93% (90%>95%), 85% (81%>89%) and 79% (73%>86%) in 2011-2018 (Figure 1C).The age-stratified multivariable analysis of RS demonstrated that RS between 2003-2010 and 2011-2018 did not improve further.
Conclusion
The use of chemotherapy alone in LPL/WM is almost entirely abandoned in contemporary clinical practice in the Netherlands. The impressive survival improvement over time may be accounted for by the increased use of rituximab-containing therapy since its introduction in early-mid 2000. The lack of survival improvement in the post-rituximab era warrant longer follow-up of our post-rituximab cohort to assess the effects of recently introduced novel agents, and should bolster forthcoming studies across various therapy lines to further improve survival in LPL/WM.
Keyword(s): Lymphoplasmacytic lymphoma, Population, Survival, Waldenstrom's macroglobulinemia
Abstract: EP796
Type: E-Poster Presentation
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
LPL/WM is a rare lymphoproliferative malignancy. Although therapeutic advances have been made in LPL/WM over the past decades, it is unclear how these advances have impacted the population-level survival of LPL/WM patients, especially among contemporary diagnosed patients. This inconclusiveness relates to the lack of population-based studies in LPL/WM.
Aims
This nationwide, population-based study aimed to assess trends in first-line therapy and relative survival (RS) among LPL/WM patients diagnosed in the Netherlands during a 30-year period.
Methods
All LPL/WM patients diagnosed between 1989-2018—with survival follow-up through 2020—were selected from the Netherlands Cancer Registry (NCR). Data on primary therapy—that is, no anti-neoplastic therapy (i.e. watch-and-wait) and anti-neoplastic therapy (e.g. chemotherapy)—were available in the NCR. Information on the use of rituximab was available for patients diagnosed as from 2007. Patients were categorized into four periods (1989-1995, 1996-2002, 2003-2010 and 2011-2018), and three age groups (<65 (n=383), 66-75 (n=384) and >75 years (n=386)). Data on the exact anti-neoplastic therapy, asymptomatic/symptomatic disease, and IPSS risk group were available for patients diagnosed as from 2014. The exact anti-neoplastic regimens were presented for patients with symptomatic disease, stratified by year of diagnosis, the three abovementioned age groups, and IPSS risk group (i.e. low, intermediate, and high). RS was calculated to estimate disease-specific survival.
Results
A total of 6,232 LPL/WM patients (median age 70 years; 61% males) were included in the study. Due to the broader application of an initial watch-and-wait approach, treatment with anti-neoplastic agents at diagnosis gradually decreased over time, irrespective of age. The proportion of patients receiving upfront anti-neoplastic therapy was 41%, 38%, and 40% across the three age groups during 2011-2018. Data from 2007 onwards showed that the use of chemotherapy alone decreased over time, following an increased application of chemoimmunotherapy (Figure 1A). Symptomatic disease was present in 60% of patients diagnosed during 2014-2018. Detailed data on primary therapy among 960 symptomatic patients revealed that DRC was the most frequently applied therapeutic regimen across all age groups, of which its use increased from 14% to 39% between 2014 and 2018. Consequently, the use of R-C(V)P decreased between 2014 and 2018. The use of anti-neoplastic therapy increased with advancing IPSS risk group (Figure 1B). Five-year RS increased significantly across all age groups between the first and last calendar period. Specifically, 5-year RS (95% confidence intervals) was 75% (69%>79%), 65% (59%>71%), and 46% (39%>53%) in 1989-1995 across the three age groups, compared with 93% (90%>95%), 85% (81%>89%) and 79% (73%>86%) in 2011-2018 (Figure 1C).The age-stratified multivariable analysis of RS demonstrated that RS between 2003-2010 and 2011-2018 did not improve further.

Conclusion
The use of chemotherapy alone in LPL/WM is almost entirely abandoned in contemporary clinical practice in the Netherlands. The impressive survival improvement over time may be accounted for by the increased use of rituximab-containing therapy since its introduction in early-mid 2000. The lack of survival improvement in the post-rituximab era warrant longer follow-up of our post-rituximab cohort to assess the effects of recently introduced novel agents, and should bolster forthcoming studies across various therapy lines to further improve survival in LPL/WM.
Keyword(s): Lymphoplasmacytic lymphoma, Population, Survival, Waldenstrom's macroglobulinemia


