
Contributions
Abstract: EP789
Type: E-Poster Presentation
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Zanu is a highly selective, potent and irreversible Bruton tyrosine kinase inhibitor approved in the United States and China for treatment of adult pts with R/R MCL. Phase 2 study results of zanu showing high activity in R/R MCL (median follow-up (mfu), 18.4 mo) were previously reported (Clin Cancer Res. 2020;26:4216). We present here long-term results of this study (mfu, 35.3 mo).
Aims
To evaluate the sustainability of efficacy and long-term safety of zanu in pts with R/R MCL.
Methods
In this single-arm, multicenter phase 2 study (NCT03206970), pts received oral zanu (160 mg twice a day) continuously until progressive disease (PD) or unacceptable toxicity. Response assessments per 2014 Lugano criteria were performed every 12 wk for 96 wk, and every 24 wk thereafter. ORR rate (investigator-assessed ≥ partial response), duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety were assessed.
Results
Eighty-six pts were enrolled at 13 centers in China. Median age was 60.5 y, and 83.7% had an intermediate-/high-risk Combined MCL International Prognostic Index score (MIPI-b). Most pts had advanced MCL (90.7% stage III/IV); 45.3% had bone marrow involvement, and 70.9% had extranodal disease. Bulky disease >10 cm and >5 cm was found in 8.1% and 43.0% of pts, respectively. Median number of prior lines of therapy was 2 (range, 1-4), and 52.3% of pts had refractory disease.
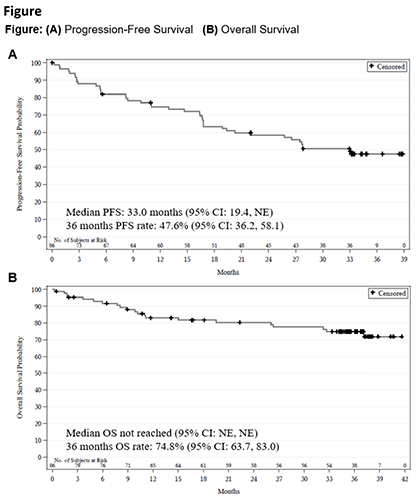
As of Sep 8, 2020, mfu was 35.3 mo (range, 0.3-41.6); 45.3% of pts were on zanu, and 54.7% had discontinued zanu primarily due to PD (43.0%) and adverse events (AEs; 9.3%). ORR was 83.7%,and 67 (77.9%) pts achieved complete response. Median DOR was not reached; 57.3% (95% CI, 44.9-67.9) of responders were estimated event (PD/death)-free at 30 mo. Median PFS was 33.0 mo (Fig A); response was generally consistent across all subgroups analyzed (MIPI-b, prior therapy, refractory status, etc). Median OS was not reached (Fig B).
The safety profile was largely unchanged vs that of the previous mfu. The most common (≥20%) treatment-emergent AEs (TEAEs) were decreased neutrophil count (46.5%), upper respiratory tract infection (38.4%), rash (36.0%), decreased white blood cell count (33.7%), and decreased platelet count (32.6%); most were grade (gr) 1/2 events. Gr ≥3 TEAEs (≥5%) were decreased neutrophil count (18.6%), pneumonia (12.8%), platelet count decreased, white blood cell count decreased (7.0% each), and anemia (5.8%). Most AEs occurred during the early stage of zanu treatment and very few new events were reported during this follow-up period. Four new pts had gr ≥3 TEAEs of any infections (18.6% total, 65.1% all gr), and no new pt had gr ≥3 TEAEs of hypertension (3.5% total, 16.3% all gr) or major hemorrhage (serious/gr ≥3 bleeding or all gr central nervous system bleeding; 3.5% total). No cases of atrial fibrillation/flutter, gr ≥3 cardiac AEs, second primary malignancies, or tumor lysis syndrome were reported throughout this study. No new TEAEs led to death (8.1% total), treatment discontinuation (9.3% total), or dose reduction (2.3% total); 3 new pts had dose interruption during this follow-up period (18.6% total).
Conclusion
These data continued to show the deep response with zanu in pts with R/R MCL that was generally consistent across all subgroups, including high-risk pts. The long-term follow-up show that sustained PFS was achieved with zanu treatment, and about half of pts remained progression free at 36 mo. The new TEAEs reported during the follow-up period were limited; there were no new safety concerns.
Keyword(s): Mantle cell lymphoma, Refractory, Relapse, Safety
Abstract: EP789
Type: E-Poster Presentation
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Zanu is a highly selective, potent and irreversible Bruton tyrosine kinase inhibitor approved in the United States and China for treatment of adult pts with R/R MCL. Phase 2 study results of zanu showing high activity in R/R MCL (median follow-up (mfu), 18.4 mo) were previously reported (Clin Cancer Res. 2020;26:4216). We present here long-term results of this study (mfu, 35.3 mo).
Aims
To evaluate the sustainability of efficacy and long-term safety of zanu in pts with R/R MCL.
Methods
In this single-arm, multicenter phase 2 study (NCT03206970), pts received oral zanu (160 mg twice a day) continuously until progressive disease (PD) or unacceptable toxicity. Response assessments per 2014 Lugano criteria were performed every 12 wk for 96 wk, and every 24 wk thereafter. ORR rate (investigator-assessed ≥ partial response), duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety were assessed.
Results
Eighty-six pts were enrolled at 13 centers in China. Median age was 60.5 y, and 83.7% had an intermediate-/high-risk Combined MCL International Prognostic Index score (MIPI-b). Most pts had advanced MCL (90.7% stage III/IV); 45.3% had bone marrow involvement, and 70.9% had extranodal disease. Bulky disease >10 cm and >5 cm was found in 8.1% and 43.0% of pts, respectively. Median number of prior lines of therapy was 2 (range, 1-4), and 52.3% of pts had refractory disease.
As of Sep 8, 2020, mfu was 35.3 mo (range, 0.3-41.6); 45.3% of pts were on zanu, and 54.7% had discontinued zanu primarily due to PD (43.0%) and adverse events (AEs; 9.3%). ORR was 83.7%,and 67 (77.9%) pts achieved complete response. Median DOR was not reached; 57.3% (95% CI, 44.9-67.9) of responders were estimated event (PD/death)-free at 30 mo. Median PFS was 33.0 mo (Fig A); response was generally consistent across all subgroups analyzed (MIPI-b, prior therapy, refractory status, etc). Median OS was not reached (Fig B).
The safety profile was largely unchanged vs that of the previous mfu. The most common (≥20%) treatment-emergent AEs (TEAEs) were decreased neutrophil count (46.5%), upper respiratory tract infection (38.4%), rash (36.0%), decreased white blood cell count (33.7%), and decreased platelet count (32.6%); most were grade (gr) 1/2 events. Gr ≥3 TEAEs (≥5%) were decreased neutrophil count (18.6%), pneumonia (12.8%), platelet count decreased, white blood cell count decreased (7.0% each), and anemia (5.8%). Most AEs occurred during the early stage of zanu treatment and very few new events were reported during this follow-up period. Four new pts had gr ≥3 TEAEs of any infections (18.6% total, 65.1% all gr), and no new pt had gr ≥3 TEAEs of hypertension (3.5% total, 16.3% all gr) or major hemorrhage (serious/gr ≥3 bleeding or all gr central nervous system bleeding; 3.5% total). No cases of atrial fibrillation/flutter, gr ≥3 cardiac AEs, second primary malignancies, or tumor lysis syndrome were reported throughout this study. No new TEAEs led to death (8.1% total), treatment discontinuation (9.3% total), or dose reduction (2.3% total); 3 new pts had dose interruption during this follow-up period (18.6% total).

Conclusion
These data continued to show the deep response with zanu in pts with R/R MCL that was generally consistent across all subgroups, including high-risk pts. The long-term follow-up show that sustained PFS was achieved with zanu treatment, and about half of pts remained progression free at 36 mo. The new TEAEs reported during the follow-up period were limited; there were no new safety concerns.
Keyword(s): Mantle cell lymphoma, Refractory, Relapse, Safety


