
Contributions
Abstract: EP787
Type: E-Poster Presentation
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
KTE-X19 is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved in the United States and European Union for the treatment of relapsed/refractory mantle cell lymphoma (MCL). In the ZUMA-2 study in which KTE-X19 was evaluated in relapsed/refractory MCL, the objective response rate at a median 17.5-month follow-up period was 92% (67% complete response rate; Blood. 2020;136[suppl, abstr]:20-22).
Aims
To report results in patients with or without progression of disease within 24 months of diagnosis (POD24), an indicator of poor outcomes (Br J Haematol. 2019;185:940-944).
Methods
Eligible patients with relapsed/refractory MCL underwent leukapheresis and conditioning chemotherapy followed by a single infusion of KTE-X19. Efficacy results are reported for the 60 treated patients with ≥1 year of follow-up (median, 17.5 months), and safety results are presented for all 68 treated patients.
Results
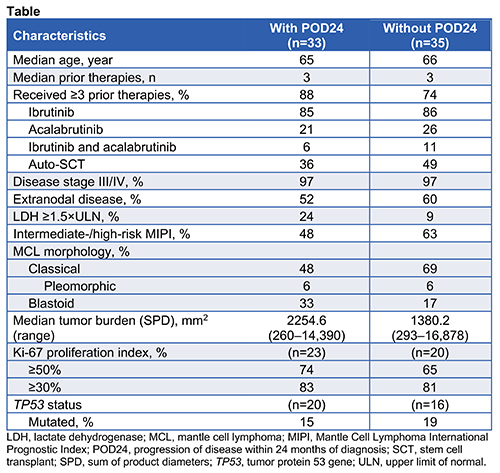
High-risk disease characteristics were common in patients with (n=33) and without POD24 (n=35), although patients with POD24 had higher tumor burden and lactate dehydrogenase levels, and more had blastoid-type MCL (Table). The objective response rate in patients with (n=28) and without POD24 (n=32) was 93% and 91%, with complete response rates of 61% and 72%, respectively. In patients with and without POD24, median progression-free survival was 11.3 months (range, 0.9–30.3) and 29.3 months (range, 0–35.9), respectively. Medians for duration of response and overall survival were not reached in either group.
Most common Grade ≥3 adverse events in patients with versus without POD24 were neutropenia (91% vs 80%), thrombocytopenia (61% vs 46%), and anemia (55% vs 51%); Grade ≥3 cytokine release syndrome and neurologic events occurred in 9% versus 20% and 27% versus 34%, respectively. There were no cases of Grade 5 cytokine release syndrome, KTE-X19–related secondary cancers, or replication-competent retrovirus in either group.
In patients with versus without POD24, median peak CAR T-cell levels and median area under the curve were 53.4 cells/mL (range, 0.2–2566) and 583.4 cells/mL (range, 1.8–27,743.6) versus 112.4 cells/mL (range, 0.2–2589) and 1588.3 cells/mL (range, 3.8–27,238.7), respectively; by 12 months, B cells were detectable in 8/11 (73%) vs 7/15 patients (47%) in ongoing response.
Conclusion
KTE-X19 provided a high complete response rate across all patients, with the medians for duration of response and overall survival not reached. Patients with POD24 had more aggressive high-risk disease characteristics (tumor burden, lactate dehydrogenase levels, and blastoid-type MCL) and generally lower CAR T-cell expansion and progression-free survival versus patients without POD24. Earlier intervention with CD19-directed CAR T-cell therapy may benefit patients with MCL with known high-risk factors.
Keyword(s): CAR-T, Clinical trial, High risk, Mantle cell lymphoma
Abstract: EP787
Type: E-Poster Presentation
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
KTE-X19 is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved in the United States and European Union for the treatment of relapsed/refractory mantle cell lymphoma (MCL). In the ZUMA-2 study in which KTE-X19 was evaluated in relapsed/refractory MCL, the objective response rate at a median 17.5-month follow-up period was 92% (67% complete response rate; Blood. 2020;136[suppl, abstr]:20-22).
Aims
To report results in patients with or without progression of disease within 24 months of diagnosis (POD24), an indicator of poor outcomes (Br J Haematol. 2019;185:940-944).
Methods
Eligible patients with relapsed/refractory MCL underwent leukapheresis and conditioning chemotherapy followed by a single infusion of KTE-X19. Efficacy results are reported for the 60 treated patients with ≥1 year of follow-up (median, 17.5 months), and safety results are presented for all 68 treated patients.
Results
High-risk disease characteristics were common in patients with (n=33) and without POD24 (n=35), although patients with POD24 had higher tumor burden and lactate dehydrogenase levels, and more had blastoid-type MCL (Table). The objective response rate in patients with (n=28) and without POD24 (n=32) was 93% and 91%, with complete response rates of 61% and 72%, respectively. In patients with and without POD24, median progression-free survival was 11.3 months (range, 0.9–30.3) and 29.3 months (range, 0–35.9), respectively. Medians for duration of response and overall survival were not reached in either group.
Most common Grade ≥3 adverse events in patients with versus without POD24 were neutropenia (91% vs 80%), thrombocytopenia (61% vs 46%), and anemia (55% vs 51%); Grade ≥3 cytokine release syndrome and neurologic events occurred in 9% versus 20% and 27% versus 34%, respectively. There were no cases of Grade 5 cytokine release syndrome, KTE-X19–related secondary cancers, or replication-competent retrovirus in either group.
In patients with versus without POD24, median peak CAR T-cell levels and median area under the curve were 53.4 cells/mL (range, 0.2–2566) and 583.4 cells/mL (range, 1.8–27,743.6) versus 112.4 cells/mL (range, 0.2–2589) and 1588.3 cells/mL (range, 3.8–27,238.7), respectively; by 12 months, B cells were detectable in 8/11 (73%) vs 7/15 patients (47%) in ongoing response.

Conclusion
KTE-X19 provided a high complete response rate across all patients, with the medians for duration of response and overall survival not reached. Patients with POD24 had more aggressive high-risk disease characteristics (tumor burden, lactate dehydrogenase levels, and blastoid-type MCL) and generally lower CAR T-cell expansion and progression-free survival versus patients without POD24. Earlier intervention with CD19-directed CAR T-cell therapy may benefit patients with MCL with known high-risk factors.
Keyword(s): CAR-T, Clinical trial, High risk, Mantle cell lymphoma


