
Contributions
Abstract: EP772
Type: E-Poster Presentation
Session title: Hodgkin lymphoma - Clinical
Background
Immunotherapy with checkpoint inhibitors (anti-PD-1) is highly effective in relapsed/refractory (R/R) classical Hodgkin Lymphoma (cHL). Nivolumab, that was registered in Russia in december 2017, is widely used for this setting as a monotherapy. However, we have promising data about using Nivolumab in combination with different chemotherapy protocols as a bridge to autologous hematopoietic stem cell transplantation (autoHSCT), which remains the standard treatment for chemosensitive R/R cHL in the past decades.
Aims
To evaluate the safety and efficacy of Nivo-DHAP combination as a bridge to autoHSCT in patients with R/R cHL.
Methods
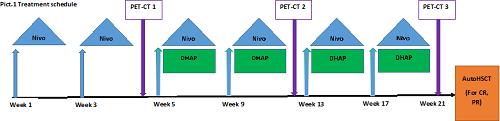
Study trial consisted of 2 cycles of Nivo as a monotherapy (fixed dose 240 mg every 14 days) with 4 subsequent cycles of Nivo-DHAP (fixed doses of Nivolumab 480 mg with standard doses of DHAP every 28 days). Response rate was assessed using PET-CT according to the Ligano and LYRIC criteria before Nivo-DHAP (PET-CT 1), after 2 cycles of Nivo-DHAP (PET-CT 2) and after 4 cycles of Nivo-DHAP (PET-CT 3). In case of chemosensitive disease at the end of the treatment, patients were proceeded to autoHSCT. Treatment schedule is represented at Picture 1. Cyclophosphamide+G-CSF, G-CSF alone or G-CSF+plerixafor were administered for stem cell mobilization. Safety assessment was carried out according to the toxicity criteria of the Common Terminology Criteria for Adverse Events (CTCAE) (v4.03). 20 patients treated from March 2020 until January 2021 were included in the preliminary analysis. All subjects provided written informed consent. Median age was 34 (21-55), male/female 12/8. 4 patients had more than 2 previous lines of therapy.
Results
The overall response rate was 75% (n=20, complete response (CR) 10%, partial response (PR) 65%, stabilization 20%, indeterminate response 5%) at PET-CT 1 (after 2 cycles of Nivo as a monotherapy). At PET-CT 2 (after 2 cycles of Nivo-DHAP) overall response rate reached 100% (n=20, CR 60%, PR 40%). At PET-CT 3 (after 4 cycles of Nivo-DHAP) it remains 100% (n=19, 1 patient is still on treatment). Hematological toxicity grade 3-4 was registered in 6 patients (30%), non-hematological toxicity were moderate (≤ grade 2): neurotoxicity – 3 (15%), nephrotoxicity – 2 (10%), skin toxicity – 1 (5%), endocrine disorders – 1(5%), infusion reactions – 1 (5%) patients respectively. Cytarabine and cisplatin doses were reduced in 5 patients (10-50% from baseline according to the standard approach). At the time of analysis, 13 patients underwent autoHSCT. In 2 patients stem cell mobilization failure was considered even with plerixafor administration.
Conclusion
Preliminary results of the combination of Nivolumab and DHAP showed high efficacy and relatively low toxicity in patients with R/R cHL before autoHSCT. We plan up to 40 patients in 2021 in order to obtain reliable data on the safety and efficacy of the regimen as well as to assess long-term survival and perform the retrospective comparative analysis with a group of patients who received the «traditional» DHAP without Nivolumab.
Keyword(s): Hodgkin's lymphoma
Abstract: EP772
Type: E-Poster Presentation
Session title: Hodgkin lymphoma - Clinical
Background
Immunotherapy with checkpoint inhibitors (anti-PD-1) is highly effective in relapsed/refractory (R/R) classical Hodgkin Lymphoma (cHL). Nivolumab, that was registered in Russia in december 2017, is widely used for this setting as a monotherapy. However, we have promising data about using Nivolumab in combination with different chemotherapy protocols as a bridge to autologous hematopoietic stem cell transplantation (autoHSCT), which remains the standard treatment for chemosensitive R/R cHL in the past decades.
Aims
To evaluate the safety and efficacy of Nivo-DHAP combination as a bridge to autoHSCT in patients with R/R cHL.
Methods
Study trial consisted of 2 cycles of Nivo as a monotherapy (fixed dose 240 mg every 14 days) with 4 subsequent cycles of Nivo-DHAP (fixed doses of Nivolumab 480 mg with standard doses of DHAP every 28 days). Response rate was assessed using PET-CT according to the Ligano and LYRIC criteria before Nivo-DHAP (PET-CT 1), after 2 cycles of Nivo-DHAP (PET-CT 2) and after 4 cycles of Nivo-DHAP (PET-CT 3). In case of chemosensitive disease at the end of the treatment, patients were proceeded to autoHSCT. Treatment schedule is represented at Picture 1. Cyclophosphamide+G-CSF, G-CSF alone or G-CSF+plerixafor were administered for stem cell mobilization. Safety assessment was carried out according to the toxicity criteria of the Common Terminology Criteria for Adverse Events (CTCAE) (v4.03). 20 patients treated from March 2020 until January 2021 were included in the preliminary analysis. All subjects provided written informed consent. Median age was 34 (21-55), male/female 12/8. 4 patients had more than 2 previous lines of therapy.
Results
The overall response rate was 75% (n=20, complete response (CR) 10%, partial response (PR) 65%, stabilization 20%, indeterminate response 5%) at PET-CT 1 (after 2 cycles of Nivo as a monotherapy). At PET-CT 2 (after 2 cycles of Nivo-DHAP) overall response rate reached 100% (n=20, CR 60%, PR 40%). At PET-CT 3 (after 4 cycles of Nivo-DHAP) it remains 100% (n=19, 1 patient is still on treatment). Hematological toxicity grade 3-4 was registered in 6 patients (30%), non-hematological toxicity were moderate (≤ grade 2): neurotoxicity – 3 (15%), nephrotoxicity – 2 (10%), skin toxicity – 1 (5%), endocrine disorders – 1(5%), infusion reactions – 1 (5%) patients respectively. Cytarabine and cisplatin doses were reduced in 5 patients (10-50% from baseline according to the standard approach). At the time of analysis, 13 patients underwent autoHSCT. In 2 patients stem cell mobilization failure was considered even with plerixafor administration.

Conclusion
Preliminary results of the combination of Nivolumab and DHAP showed high efficacy and relatively low toxicity in patients with R/R cHL before autoHSCT. We plan up to 40 patients in 2021 in order to obtain reliable data on the safety and efficacy of the regimen as well as to assess long-term survival and perform the retrospective comparative analysis with a group of patients who received the «traditional» DHAP without Nivolumab.
Keyword(s): Hodgkin's lymphoma


