
Contributions
Abstract: EP771
Type: E-Poster Presentation
Session title: Hodgkin lymphoma - Clinical
Background
Currently, the issue of adequate criteria for evaluation of response to immunotherapy of Hodgkin lymphoma is not resolved. LYmphoma Response to Immunomodulatory therapy Criteria (LYRIC) introduced a new concept of indeterminate response (IR). However, only a limited number of studies have evaluated the efficacy of PD-1 inhibitors using LYRIC criteria. Thus, the prognosis of patients who develop IR warrants further analysis.
Aims
To assess the response evolution and prognosis of patients with relapsed and refractory classical Hodgkin lymphoma (r/r cHL) who demonstrated IR during nivolumab (Nivo) therapy.
Methods
We retrospective reviewed data on 160 patients with r/r cHL treated with Nivo 3 mg/kg as part of the Russian Named Patient Program or 40 mg as part of a clinical trial (NCT03343665). Response was evaluated using PET-CT every 6 cycles (3 months).
The analysis identified 56 (35%) patients who achieved IR at first response evaluation according to LYRIC criteria after 3 months of therapy. Nivo dose was 3 mg/kg in 39 (70%) patients, 40 mg in 17 (30%) pts. There were 25 (45%) male and 31 (55%) female patients. Median age was 36 (23-64). Median time from diagnosis to Nivo was 33 (10-200) months. Thirty-nine (70%) patients were resistant to first-line chemotherapy. Prior to Nivo treatment brentuximab vedotin was administered in 24 (43%) patients and 15 (27%) patients underwent autologous HSCT. Stable disease (SD), partial response (PR) and progressive disease (PD) were in 1 (2%), 9 (16%) and 46 (82%) pts respectively. Extranodal disease was detected in 41 (73%) pts and B-symptoms in 37 (66%) pts. Median number of therapy lines was 4 (2-10).
Results
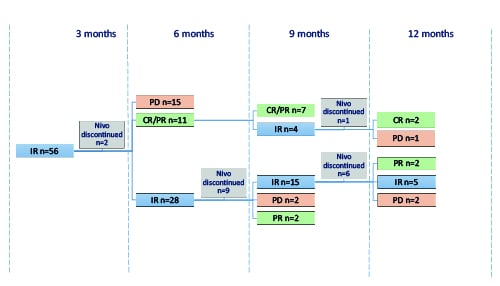
Two out of 56 pts discontinued Nivo at 3 months of therapy due to patient’s decision. After 6 months of Nivo therapy 28 pts maintained IR, other variants of response are demonstrated in Picture 1. Nine patients with IR at 6 months discontinued Nivo due to discretion of the treating physician and continued different type of therapy. Among 19 pts with IR who continued Nivo therapy, at 9 months of treatment 15 pts retained IR. After 9 months of therapy Nivo was discontinued in 6 pts with IR due to discretion of the treating physician in 4 pts, secondary MDS in 1 pt and Named Patient Program closure in 1 pt. At 12 months of therapy 5 pts maintained IR, 2 pts transformed to PR and 2 pts to PD [Pic.1].
It is worth noting that biopsy was performed at the time of IR in 3 pts and tumor cells were found in all specimens.
Median follow-up was 36 (6-53) months. Three-year overall survival (OS) and progression-free survival (PFS) was 93% and 20%, respectively. Nivo dose was not associated with OS (p=0.76) or PFS (p=0.11). Disease status at the Nivo initiation was significantly associated with PFS (p=0.04), but not with OS. Extranodal disease presence was significantly associated with PFS (p<0.0001), but not with OS.
Conclusion
Patients with r/r cHL can maintain long-term stable clinical course during IR and Nivo therapy continuation despite the presence of tumor cells revealed in biopsy specimens. Thus, LYRIC criteria allow preventing early discontinuation of PD-1 inhibitor therapy in patients for which it could be potentially beneficial.
Keyword(s): Hodgkin's disease, Immune therapy, Refractory, Relapsed lymphoma
Abstract: EP771
Type: E-Poster Presentation
Session title: Hodgkin lymphoma - Clinical
Background
Currently, the issue of adequate criteria for evaluation of response to immunotherapy of Hodgkin lymphoma is not resolved. LYmphoma Response to Immunomodulatory therapy Criteria (LYRIC) introduced a new concept of indeterminate response (IR). However, only a limited number of studies have evaluated the efficacy of PD-1 inhibitors using LYRIC criteria. Thus, the prognosis of patients who develop IR warrants further analysis.
Aims
To assess the response evolution and prognosis of patients with relapsed and refractory classical Hodgkin lymphoma (r/r cHL) who demonstrated IR during nivolumab (Nivo) therapy.
Methods
We retrospective reviewed data on 160 patients with r/r cHL treated with Nivo 3 mg/kg as part of the Russian Named Patient Program or 40 mg as part of a clinical trial (NCT03343665). Response was evaluated using PET-CT every 6 cycles (3 months).
The analysis identified 56 (35%) patients who achieved IR at first response evaluation according to LYRIC criteria after 3 months of therapy. Nivo dose was 3 mg/kg in 39 (70%) patients, 40 mg in 17 (30%) pts. There were 25 (45%) male and 31 (55%) female patients. Median age was 36 (23-64). Median time from diagnosis to Nivo was 33 (10-200) months. Thirty-nine (70%) patients were resistant to first-line chemotherapy. Prior to Nivo treatment brentuximab vedotin was administered in 24 (43%) patients and 15 (27%) patients underwent autologous HSCT. Stable disease (SD), partial response (PR) and progressive disease (PD) were in 1 (2%), 9 (16%) and 46 (82%) pts respectively. Extranodal disease was detected in 41 (73%) pts and B-symptoms in 37 (66%) pts. Median number of therapy lines was 4 (2-10).
Results
Two out of 56 pts discontinued Nivo at 3 months of therapy due to patient’s decision. After 6 months of Nivo therapy 28 pts maintained IR, other variants of response are demonstrated in Picture 1. Nine patients with IR at 6 months discontinued Nivo due to discretion of the treating physician and continued different type of therapy. Among 19 pts with IR who continued Nivo therapy, at 9 months of treatment 15 pts retained IR. After 9 months of therapy Nivo was discontinued in 6 pts with IR due to discretion of the treating physician in 4 pts, secondary MDS in 1 pt and Named Patient Program closure in 1 pt. At 12 months of therapy 5 pts maintained IR, 2 pts transformed to PR and 2 pts to PD [Pic.1].
It is worth noting that biopsy was performed at the time of IR in 3 pts and tumor cells were found in all specimens.
Median follow-up was 36 (6-53) months. Three-year overall survival (OS) and progression-free survival (PFS) was 93% and 20%, respectively. Nivo dose was not associated with OS (p=0.76) or PFS (p=0.11). Disease status at the Nivo initiation was significantly associated with PFS (p=0.04), but not with OS. Extranodal disease presence was significantly associated with PFS (p<0.0001), but not with OS.

Conclusion
Patients with r/r cHL can maintain long-term stable clinical course during IR and Nivo therapy continuation despite the presence of tumor cells revealed in biopsy specimens. Thus, LYRIC criteria allow preventing early discontinuation of PD-1 inhibitor therapy in patients for which it could be potentially beneficial.
Keyword(s): Hodgkin's disease, Immune therapy, Refractory, Relapsed lymphoma


