
Contributions
Abstract: EP766
Type: E-Poster Presentation
Session title: Hodgkin lymphoma - Clinical
Background
Patients with relapsed/refractory classical Hodgkin lymphoma (r/r cHL) generally have a poor prognosis. We have shown that brentuximab vedotin (BV) in combination with DHAP (dexamethasone, cytarabine, cisplatin) is highly effective with manageable toxicity. Here we present the long-term follow-up, including thymus and activation regulated chemokine (TARC) and metabolic tumor volume (MTV) analyses.
Aims
The aim was to find predictive biomarkers for r/r cHL patients treated with BV-DHAP and to evaluate the long-term follow-up.
Methods
Patients were treated with 3 cycles of BV-DHAP followed by autologous stem-cell transplant (ASCT). Serum was collected at baseline, after each cycle, and during follow-up. Soluble (s)TARC was measured by ELISA. Based on levels in healthy controls, a baseline sTARC <1000 pg/mL was considered not elevated. Immunohistochemical (IHC) staining was done for TARC on the lymph node biopsy at baseline. Baseline 18F-FDG-PET-CT scans were analyzed quantitatively for MTV using a fixed threshold of standard uptake value (SUV) ≥4.0. Predictive value of biomarkers for time to progression (TTP) was assessed by calculating the area under the curve (AUC) of the receiver operating characteristics curve and log-rank survival analysis. For biomarker evaluation, patients who died without progression (n=2) were censored at time of death.
Results
Sixty-five patients, of whom 46% had primary refractory disease, were included. Central pathology review confirmed cHL diagnosis in 58/65 patients. Patients with other histologies (e.g. peripheral T-cell lymphoma) were excluded from biomarker analyses, but not from clinical evaluation per intention to treat. After a median follow-up of 39 months, the 3-year PFS was 77% (95%CI: 70-88) and the OS was 95% (95%CI: 90-100).
Of confirmed cHL patients (n=58), seven patients (12%) had weak or no TARC IHC staining in the Hodgkin-Reed Sternberg (HRS) cells and showed lower sTARC levels compared to patients with positive TARC staining (median 608 vs 3701 pg/mL; p=0.04). cHL patients with no/weak TARC staining had a lower 3-year TTP (57% vs 93%, respectively; p<0.001).
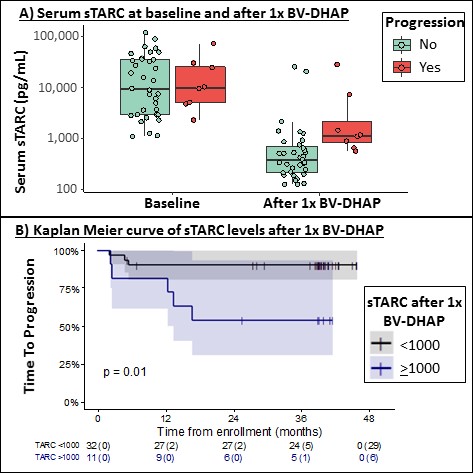
Baseline sTARC was not predictive for TTP (AUC 0.47). cHL patients with a baseline sTARC <1000 pg/mL (n=14) were excluded in the subsequent sTARC analyses. sTARC decreased significantly after 1 cycle of BV-DHAP (p<0.001; Figure 1A) and patients with sTARC <1000 pg/mL after 1 cycle of BV-DHAP had a 3-year TTP of 91% vs 55% for patients with sTARC ≥1000 pg/mL (p=0.01; AUC 0.84; Figure 1B).
Baseline MTV correlated with baseline sTARC (r=0.53; p<0.001) but did not predict TTP (AUC 0.49). On the interimPET after 3 cycles of BV-DHAP, 5 patients (8%) had a partial response (PR; Deauville 4-5) and these patients had a 3-year TTP of 40% vs 93% for patients with a complete metabolic response (n=47; 90%; p<0.001). Patients with PR who had progression after ASCT (n=3) showed higher sTARC levels at time of the interimPET compared to the 2 patients with PR who did not progress after ASCT (mean 1730 vs 194 pg/mL; p=0.06, respectively).
Conclusion
Treatment with BV-DHAP in r/r cHL patients shows a high 3-year PFS and OS. Serum sTARC correlates with baseline MTV and can be used as an early response marker already after 1 cycle of BV-DHAP. InterimPET status is predictive for post-ASCT TTP and sTARC can complement interimPET response evaluation. In addition, we have identified low TARC expression in HRS cells as a possible poor prognostic factor. Risk assessment using different biomarkers could help guide treatment decisions in an era with new targeted treatment options for r/r cHL.
Keyword(s): Follow-up, Hodgkin's lymphoma, Prognostic factor, Targeted therapy
Abstract: EP766
Type: E-Poster Presentation
Session title: Hodgkin lymphoma - Clinical
Background
Patients with relapsed/refractory classical Hodgkin lymphoma (r/r cHL) generally have a poor prognosis. We have shown that brentuximab vedotin (BV) in combination with DHAP (dexamethasone, cytarabine, cisplatin) is highly effective with manageable toxicity. Here we present the long-term follow-up, including thymus and activation regulated chemokine (TARC) and metabolic tumor volume (MTV) analyses.
Aims
The aim was to find predictive biomarkers for r/r cHL patients treated with BV-DHAP and to evaluate the long-term follow-up.
Methods
Patients were treated with 3 cycles of BV-DHAP followed by autologous stem-cell transplant (ASCT). Serum was collected at baseline, after each cycle, and during follow-up. Soluble (s)TARC was measured by ELISA. Based on levels in healthy controls, a baseline sTARC <1000 pg/mL was considered not elevated. Immunohistochemical (IHC) staining was done for TARC on the lymph node biopsy at baseline. Baseline 18F-FDG-PET-CT scans were analyzed quantitatively for MTV using a fixed threshold of standard uptake value (SUV) ≥4.0. Predictive value of biomarkers for time to progression (TTP) was assessed by calculating the area under the curve (AUC) of the receiver operating characteristics curve and log-rank survival analysis. For biomarker evaluation, patients who died without progression (n=2) were censored at time of death.
Results
Sixty-five patients, of whom 46% had primary refractory disease, were included. Central pathology review confirmed cHL diagnosis in 58/65 patients. Patients with other histologies (e.g. peripheral T-cell lymphoma) were excluded from biomarker analyses, but not from clinical evaluation per intention to treat. After a median follow-up of 39 months, the 3-year PFS was 77% (95%CI: 70-88) and the OS was 95% (95%CI: 90-100).
Of confirmed cHL patients (n=58), seven patients (12%) had weak or no TARC IHC staining in the Hodgkin-Reed Sternberg (HRS) cells and showed lower sTARC levels compared to patients with positive TARC staining (median 608 vs 3701 pg/mL; p=0.04). cHL patients with no/weak TARC staining had a lower 3-year TTP (57% vs 93%, respectively; p<0.001).
Baseline sTARC was not predictive for TTP (AUC 0.47). cHL patients with a baseline sTARC <1000 pg/mL (n=14) were excluded in the subsequent sTARC analyses. sTARC decreased significantly after 1 cycle of BV-DHAP (p<0.001; Figure 1A) and patients with sTARC <1000 pg/mL after 1 cycle of BV-DHAP had a 3-year TTP of 91% vs 55% for patients with sTARC ≥1000 pg/mL (p=0.01; AUC 0.84; Figure 1B).
Baseline MTV correlated with baseline sTARC (r=0.53; p<0.001) but did not predict TTP (AUC 0.49). On the interimPET after 3 cycles of BV-DHAP, 5 patients (8%) had a partial response (PR; Deauville 4-5) and these patients had a 3-year TTP of 40% vs 93% for patients with a complete metabolic response (n=47; 90%; p<0.001). Patients with PR who had progression after ASCT (n=3) showed higher sTARC levels at time of the interimPET compared to the 2 patients with PR who did not progress after ASCT (mean 1730 vs 194 pg/mL; p=0.06, respectively).

Conclusion
Treatment with BV-DHAP in r/r cHL patients shows a high 3-year PFS and OS. Serum sTARC correlates with baseline MTV and can be used as an early response marker already after 1 cycle of BV-DHAP. InterimPET status is predictive for post-ASCT TTP and sTARC can complement interimPET response evaluation. In addition, we have identified low TARC expression in HRS cells as a possible poor prognostic factor. Risk assessment using different biomarkers could help guide treatment decisions in an era with new targeted treatment options for r/r cHL.
Keyword(s): Follow-up, Hodgkin's lymphoma, Prognostic factor, Targeted therapy


