
Contributions
Abstract: EP765
Type: E-Poster Presentation
Session title: Hodgkin lymphoma - Clinical
Background
In the treatment of advanced Hodgkin lymphoma, it is increasingly common practice to modify escalated BEACOPP (eBPP) by removing oral procarbazine and replacing it with intravenous dacarbazine (250mg/m2 D2-3) to reduce haematopoietic stem cell and gonadal toxicity. However, published data of the 'escalated BEACOPDac (eBPDac)' regimen are very limited.
Aims
The aims are to compare efficacy and toxicity outcomes of patients treated with eBPDac versus eBPP.
Methods
This is a retrospective study of 147 patients from 16 centres in the UK, Ireland and France who were treated with eBPDac first line for advanced stage Hodgkin lymphoma. Outcomes were compared with 58 matched patients treated with eBPP at 4 UK centres. Most patients were treated as per HD15 or HD18 protocol. 28 patients in Paris and two in Truro followed the AHL2011 protocol with 2 courses of eBPDac given upfront and if iPET2 negative were deescalated to 4 cycles of ABVD.
Results
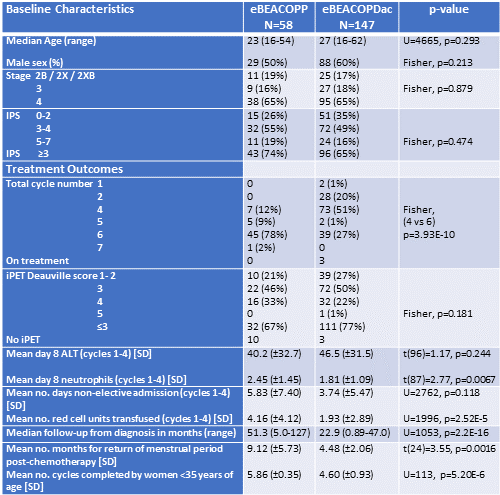
From 2009, 205 patients were treated first line with either eBPP (n=58) or eBPDac (n=147) with median follow-up 51.3 months and 22.9 months respectively. Patients were well matched with no significant differences in age (median: 23 y vs 27 y), sex, stage (stage 3/4: 81% vs 83%) and international prognostic score (IPS3+: 74% vs 65%).
51% of eBPDac patients received only 4 cycles (vs 12% of eBPP patients; p<0.001) reflecting publication of HD18 trial data. In total, 74% patients achieved iPET2 Deauville score ≤3 and 90% patients achieved PET negative remission by end of treatment. 77% of eBPDac patients achieved iPET2 Deauville ≤3 which was statistically similar to the eBPP cohort (67%; p=0.181) and matched the 76% iPET D2/3 reported in HD18. Of 205 patients, 202 are alive and 197 continue in first remission. Two eBPP patients have relapsed at 13 and 41 months and the latter died of refractory disease. One eBPDac patient had primary refractory disease, and three have relapsed at 2, 7 and 24 months. One 56-year-old eBPDac patient with high IPS died with bowel perforation during cycle 1 and one 34-year-old with alcoholic liver disease died 8 months after treatment while still in remission.
Toxicity was compared over the first 4 cycles. Mean day 8 (D8) ALT was similar between the two regimens. Mean D8 neutrophil count was lower in eBPDac than eBPP patients (1.81 vs 2.45; p=0.067; G-CSF given day 9), however it increased to 5.61 in eBPDac patients given GCSF from day 4. There is a trend toward fewer non-elective days of inpatient care for eBPDac compared with eBPP (mean: 3.74 vs 5.83; p=0.118), and eBPDac patients received fewer red cell transfusions compared with eBPP patients (mean 1.93 units vs 4.16 units; p<0.001). Women aged < 35, who completed ≥4 cycles of eBPDac/eBPP had a similar rate of return of menstrual cycles (eBPP: 22/25; eBPDac: 29/29), although eBPDac patients appeared to restart menstruation earlier post chemotherapy (mean: 4.48 months vs 9.12 months, p=0.0026). However, this could also reflect the higher mean chemotherapy cycle number completed by the eBPP women (5.86 vs 4.60; p<0.001). The use of Goserelin to suppress ovulation varied between centres.
Conclusion
Accepting the limitations of a retrospective study, we suggest that substituting dacarbazine for procarbazine is unlikely to compromise the efficacy of eBPP and may have some toxicity benefits. Despite a predominance of high risk advanced stage patients, with nearly 2 years median follow-up we have observed only 2 deaths and 4 progression events from 147 patients treated with eBPDac, suggesting this regimen is highly efficacious for the treatment of Hodgkin lymphoma.
Keyword(s): Hodgkin's lymphoma, Toxicity
Abstract: EP765
Type: E-Poster Presentation
Session title: Hodgkin lymphoma - Clinical
Background
In the treatment of advanced Hodgkin lymphoma, it is increasingly common practice to modify escalated BEACOPP (eBPP) by removing oral procarbazine and replacing it with intravenous dacarbazine (250mg/m2 D2-3) to reduce haematopoietic stem cell and gonadal toxicity. However, published data of the 'escalated BEACOPDac (eBPDac)' regimen are very limited.
Aims
The aims are to compare efficacy and toxicity outcomes of patients treated with eBPDac versus eBPP.
Methods
This is a retrospective study of 147 patients from 16 centres in the UK, Ireland and France who were treated with eBPDac first line for advanced stage Hodgkin lymphoma. Outcomes were compared with 58 matched patients treated with eBPP at 4 UK centres. Most patients were treated as per HD15 or HD18 protocol. 28 patients in Paris and two in Truro followed the AHL2011 protocol with 2 courses of eBPDac given upfront and if iPET2 negative were deescalated to 4 cycles of ABVD.
Results
From 2009, 205 patients were treated first line with either eBPP (n=58) or eBPDac (n=147) with median follow-up 51.3 months and 22.9 months respectively. Patients were well matched with no significant differences in age (median: 23 y vs 27 y), sex, stage (stage 3/4: 81% vs 83%) and international prognostic score (IPS3+: 74% vs 65%).
51% of eBPDac patients received only 4 cycles (vs 12% of eBPP patients; p<0.001) reflecting publication of HD18 trial data. In total, 74% patients achieved iPET2 Deauville score ≤3 and 90% patients achieved PET negative remission by end of treatment. 77% of eBPDac patients achieved iPET2 Deauville ≤3 which was statistically similar to the eBPP cohort (67%; p=0.181) and matched the 76% iPET D2/3 reported in HD18. Of 205 patients, 202 are alive and 197 continue in first remission. Two eBPP patients have relapsed at 13 and 41 months and the latter died of refractory disease. One eBPDac patient had primary refractory disease, and three have relapsed at 2, 7 and 24 months. One 56-year-old eBPDac patient with high IPS died with bowel perforation during cycle 1 and one 34-year-old with alcoholic liver disease died 8 months after treatment while still in remission.
Toxicity was compared over the first 4 cycles. Mean day 8 (D8) ALT was similar between the two regimens. Mean D8 neutrophil count was lower in eBPDac than eBPP patients (1.81 vs 2.45; p=0.067; G-CSF given day 9), however it increased to 5.61 in eBPDac patients given GCSF from day 4. There is a trend toward fewer non-elective days of inpatient care for eBPDac compared with eBPP (mean: 3.74 vs 5.83; p=0.118), and eBPDac patients received fewer red cell transfusions compared with eBPP patients (mean 1.93 units vs 4.16 units; p<0.001). Women aged < 35, who completed ≥4 cycles of eBPDac/eBPP had a similar rate of return of menstrual cycles (eBPP: 22/25; eBPDac: 29/29), although eBPDac patients appeared to restart menstruation earlier post chemotherapy (mean: 4.48 months vs 9.12 months, p=0.0026). However, this could also reflect the higher mean chemotherapy cycle number completed by the eBPP women (5.86 vs 4.60; p<0.001). The use of Goserelin to suppress ovulation varied between centres.

Conclusion
Accepting the limitations of a retrospective study, we suggest that substituting dacarbazine for procarbazine is unlikely to compromise the efficacy of eBPP and may have some toxicity benefits. Despite a predominance of high risk advanced stage patients, with nearly 2 years median follow-up we have observed only 2 deaths and 4 progression events from 147 patients treated with eBPDac, suggesting this regimen is highly efficacious for the treatment of Hodgkin lymphoma.
Keyword(s): Hodgkin's lymphoma, Toxicity


