
Contributions
Abstract: EP757
Type: E-Poster Presentation
Session title: Hematopoiesis, stem cells and microenvironment
Background
Clonal hematopoiesis of indeterminate potential (CHIP) is driven by leukemia-associated somatic variants, primarily affecting the genes DNMT3A, TET2, ASXL1 and JAK2 and rises steadily with age. While CHIP occurs among the healthy elderly population, CHIP is associated with several pathogenic states, including an elevated risk for hematologic neoplasms, ischemic stroke and overall mortality. Experimental evidence suggests that CHIP is a novel emerging causal cardiovascular risk factor that contributes to the development of atherosclerosis and cardiac dysfunction and exacerbates cardiovascular diseases. Recent studies report an association of CHIP with ischemic heart failure as well as degenerative aortic valve stenosis resulting in increased mortality.
Aims
The goal of our study is to characterize the prevalence and clinical impact of CHIP in patients undergoing heart transplantation.
Methods
We investigated 82 patients who underwent heart transplantation between 2013 and 2018 in the Department of Cardiac, Thoracic, Transplant and Vascular Surgery of Hannover Medical School, and for whom DNA from peripheral blood before heart transplantation and follow up information was available. The study was approved by the institutional review board of Hannover Medical School and written informed consent was obtained according to the Declaration of Helsinki. DNA from whole blood was evaluated for CHIP with a custom TruSight myeloid sequencing panel and next generation sequencing (NGS), including 46 entire genes or hotspots. Variants were validated by an established NGS-MRD method with a sensitivity of 0.02%.
Results
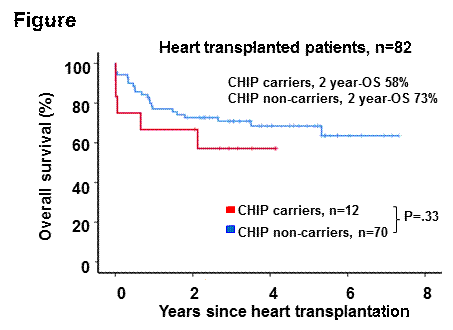
Of 82 patients with a median age of 55.5 years, 39 (47.6%) were diagnosed with a dilated cardiomyopathy (DCM), 24 (29.3%) with coronary artery disease (CAD), 7 (8.5%) with restrictive cardiomyopathy (RCM) and 12 (14.6%) with other heart diseases. Fifty-six patients were male (68.3%). Twelve of 82 (14.6%) patients carried CHIP-associated variants. The median variant allele frequency was 9.76% (range 3.06-32.7%). One of the 12 patients carried 3 and one patient carried 2 concurrent CHIP-associated variants. Mutations in DNMT3A were the most frequently detected and were observed in 6 of 12 patients (50%). The prevalence of CHIP in DCM, CAD, RCM and other patients was 25.5, 12.5, 0 and 9.1%, respectively (P=.46). CHIP carriers were numerically older compared to non-carriers (59 vs 53.5 years, P=0.4) and had numerically less cardiovascular risk factors (CVRFs) at the time of HTx (>1 CVRF 75% vs 90%, P=.16; >2 CVRFs 16.7 vs 35.7%, P=.09). All other investigated baseline characteristics (hemoglobin, platelet count, leukocytes, donor/recipient characteristics) for HTx did not differ between the groups. Day-90 mortality was 25% vs 5.7% in CHIP carriers vs non-carriers (P.27). CHIP carriers had a similar 2-year overall survival (OS) after HTx when compared to non-carriers (58% vs 73%, P=.33, HR 1.62, 95%CI 0.61-4.3, Figure). CHIP-associated variants had no impact on the risk of allograft rejection (77.1% vs 58.3%, P=.28) or malignancy development after HTx (11.4% vs 16.7%, P=.64) compared to CHIP non-carriers.
Conclusion
Acquired somatic mutations in the CHIP-driver genes occur with a prevalence of 14.6% in patients undergoing HTx. Patients with CHIP had fewer traditional cardiovascular risk factors, suggesting that CHIP may contribute to their cardiovascular risk. CHIP was associated with a higher early mortality rate but did not affect long-term survival in patients undergoing HTx.
Axel Haverich and Michael Heuser share the senior authorship.
Keyword(s): Clonality, Transplant-related mortality
Abstract: EP757
Type: E-Poster Presentation
Session title: Hematopoiesis, stem cells and microenvironment
Background
Clonal hematopoiesis of indeterminate potential (CHIP) is driven by leukemia-associated somatic variants, primarily affecting the genes DNMT3A, TET2, ASXL1 and JAK2 and rises steadily with age. While CHIP occurs among the healthy elderly population, CHIP is associated with several pathogenic states, including an elevated risk for hematologic neoplasms, ischemic stroke and overall mortality. Experimental evidence suggests that CHIP is a novel emerging causal cardiovascular risk factor that contributes to the development of atherosclerosis and cardiac dysfunction and exacerbates cardiovascular diseases. Recent studies report an association of CHIP with ischemic heart failure as well as degenerative aortic valve stenosis resulting in increased mortality.
Aims
The goal of our study is to characterize the prevalence and clinical impact of CHIP in patients undergoing heart transplantation.
Methods
We investigated 82 patients who underwent heart transplantation between 2013 and 2018 in the Department of Cardiac, Thoracic, Transplant and Vascular Surgery of Hannover Medical School, and for whom DNA from peripheral blood before heart transplantation and follow up information was available. The study was approved by the institutional review board of Hannover Medical School and written informed consent was obtained according to the Declaration of Helsinki. DNA from whole blood was evaluated for CHIP with a custom TruSight myeloid sequencing panel and next generation sequencing (NGS), including 46 entire genes or hotspots. Variants were validated by an established NGS-MRD method with a sensitivity of 0.02%.
Results
Of 82 patients with a median age of 55.5 years, 39 (47.6%) were diagnosed with a dilated cardiomyopathy (DCM), 24 (29.3%) with coronary artery disease (CAD), 7 (8.5%) with restrictive cardiomyopathy (RCM) and 12 (14.6%) with other heart diseases. Fifty-six patients were male (68.3%). Twelve of 82 (14.6%) patients carried CHIP-associated variants. The median variant allele frequency was 9.76% (range 3.06-32.7%). One of the 12 patients carried 3 and one patient carried 2 concurrent CHIP-associated variants. Mutations in DNMT3A were the most frequently detected and were observed in 6 of 12 patients (50%). The prevalence of CHIP in DCM, CAD, RCM and other patients was 25.5, 12.5, 0 and 9.1%, respectively (P=.46). CHIP carriers were numerically older compared to non-carriers (59 vs 53.5 years, P=0.4) and had numerically less cardiovascular risk factors (CVRFs) at the time of HTx (>1 CVRF 75% vs 90%, P=.16; >2 CVRFs 16.7 vs 35.7%, P=.09). All other investigated baseline characteristics (hemoglobin, platelet count, leukocytes, donor/recipient characteristics) for HTx did not differ between the groups. Day-90 mortality was 25% vs 5.7% in CHIP carriers vs non-carriers (P.27). CHIP carriers had a similar 2-year overall survival (OS) after HTx when compared to non-carriers (58% vs 73%, P=.33, HR 1.62, 95%CI 0.61-4.3, Figure). CHIP-associated variants had no impact on the risk of allograft rejection (77.1% vs 58.3%, P=.28) or malignancy development after HTx (11.4% vs 16.7%, P=.64) compared to CHIP non-carriers.

Conclusion
Acquired somatic mutations in the CHIP-driver genes occur with a prevalence of 14.6% in patients undergoing HTx. Patients with CHIP had fewer traditional cardiovascular risk factors, suggesting that CHIP may contribute to their cardiovascular risk. CHIP was associated with a higher early mortality rate but did not affect long-term survival in patients undergoing HTx.
Axel Haverich and Michael Heuser share the senior authorship.
Keyword(s): Clonality, Transplant-related mortality


