
Contributions
Abstract: EP756
Type: E-Poster Presentation
Session title: Hematopoiesis, stem cells and microenvironment
Background
In patients with hemoblastosis, the stromal microenvironment of the bone marrow (BM) is changed, and there is convincing evidence supporting the role of multipotent mesenchymal stromal cells (MSCs) in the transformation of the bone marrow niche, which, as a result, promotes the survival of leukemic cells. MSCs regulate both normal and malignant hematopoiesis. The preservation of leukemia stem cells (LSC) in the body even after high-dose chemotherapy is the main difficulty in the treatment of hemoblastosis, which determines the development of relapses. Since LSCs are located in their niche and are regulated by various non-hematopoietic cells, including MSCs, studying the mechanisms of interaction between LSCs and MSCs can increase the effectiveness of treatment.
Aims
The aim of this work was to study general changes and differences in the characteristics of MSCs in patients with myeloid leukemia and patients with lymphoproliferative diseases.
Methods
The study included MSCs and colony-forming units fibroblast (CFU-F) obtained from the BM of 114 patients in the onset of the disease: 32 with AML (13 male and 20 female, age 39.2±2.3) 20-ALL (7 male and 13 female, age 26.4±1.9), 20-CML (11 male and 9 female, age 44.6±3.8) and 41-DLBCL (15 male and 26 female, age 54.9±2,0). MSCs and CFU-F from the BM of donors of appropriate age were used as control. All donors and patients signed informed consent. MSCs and CFU-F were cultured by standard methods. Relative expression level (REL) of several genes were investigated by real time PCR.
Results
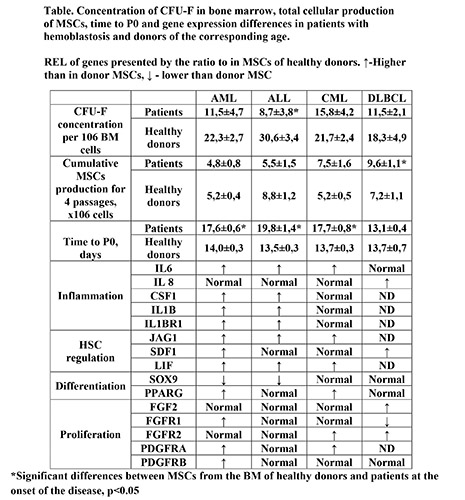
The concentration of CFU-F in the BM of patients of all studied hemoblastosis at the onset of the disease is reduced in comparison with the values in donors of the corresponding age (Table). At the same time, concentration of CFU-F in the BM of ALL patients was most pronounced and reliably reduced by almost 4 times. A decrease in the concentration of these stromal progenitor cells may reflect systemic damage to the BM stroma. The revealed changes in DLBCL patients without bone marrow involvement indicate that there is not only a direct effect of leukemic cells on the stroma due to intercellular contacts, but also an indirect one, through factors secreted by the tumor. In patients with DLBCL total MSCs production is significantly higher than in donors. In other patients cumulative MSCs production does not differ from donors. In stromal cells of patients with hemoblastosis, there are both general changes associated with proliferation, differentiation and maintenance of hematopoiesis, as well as fundamental differences presented in Table. In all patients MSCs REL of SPP1, ICAM1, TGFb1, and TGFb2 genes did not differ from donors’ ones. In the MSCs of patients with leukemia, the REL of IL6, JAG1, LIF genes were increased, and REL of IL8, SDF1 were not changed compared to the MSCs of the donors. MSCs from patients with acute leukemia were characterized by a decrease in SOX9 and an increase in CSF1, IL1b, and IL1bR1 expression. In MSCs of patients with myeloid leukemia, the PPARG and PDGFRA were increased. Gene expression in MSCs from DLBCL patients was significantly different from that in patients with leukemia.
Conclusion
Altered functions of the stromal microenvironment in patients indicate its role in the maintenance and proliferation of tumor cells. The findings could pave the way for new therapies that target the altered stromal niche for hematopoietic cells.
The materials supported by grants from the Russian Foundation for Basic Research project 17-00-00170 and 19-29-04023 were used.
Keyword(s): Acute leukemia, Chronic myeloid leukemia, Diffuse large B cell lymphoma, Mesenchymal stem cell
Abstract: EP756
Type: E-Poster Presentation
Session title: Hematopoiesis, stem cells and microenvironment
Background
In patients with hemoblastosis, the stromal microenvironment of the bone marrow (BM) is changed, and there is convincing evidence supporting the role of multipotent mesenchymal stromal cells (MSCs) in the transformation of the bone marrow niche, which, as a result, promotes the survival of leukemic cells. MSCs regulate both normal and malignant hematopoiesis. The preservation of leukemia stem cells (LSC) in the body even after high-dose chemotherapy is the main difficulty in the treatment of hemoblastosis, which determines the development of relapses. Since LSCs are located in their niche and are regulated by various non-hematopoietic cells, including MSCs, studying the mechanisms of interaction between LSCs and MSCs can increase the effectiveness of treatment.
Aims
The aim of this work was to study general changes and differences in the characteristics of MSCs in patients with myeloid leukemia and patients with lymphoproliferative diseases.
Methods
The study included MSCs and colony-forming units fibroblast (CFU-F) obtained from the BM of 114 patients in the onset of the disease: 32 with AML (13 male and 20 female, age 39.2±2.3) 20-ALL (7 male and 13 female, age 26.4±1.9), 20-CML (11 male and 9 female, age 44.6±3.8) and 41-DLBCL (15 male and 26 female, age 54.9±2,0). MSCs and CFU-F from the BM of donors of appropriate age were used as control. All donors and patients signed informed consent. MSCs and CFU-F were cultured by standard methods. Relative expression level (REL) of several genes were investigated by real time PCR.
Results
The concentration of CFU-F in the BM of patients of all studied hemoblastosis at the onset of the disease is reduced in comparison with the values in donors of the corresponding age (Table). At the same time, concentration of CFU-F in the BM of ALL patients was most pronounced and reliably reduced by almost 4 times. A decrease in the concentration of these stromal progenitor cells may reflect systemic damage to the BM stroma. The revealed changes in DLBCL patients without bone marrow involvement indicate that there is not only a direct effect of leukemic cells on the stroma due to intercellular contacts, but also an indirect one, through factors secreted by the tumor. In patients with DLBCL total MSCs production is significantly higher than in donors. In other patients cumulative MSCs production does not differ from donors. In stromal cells of patients with hemoblastosis, there are both general changes associated with proliferation, differentiation and maintenance of hematopoiesis, as well as fundamental differences presented in Table. In all patients MSCs REL of SPP1, ICAM1, TGFb1, and TGFb2 genes did not differ from donors’ ones. In the MSCs of patients with leukemia, the REL of IL6, JAG1, LIF genes were increased, and REL of IL8, SDF1 were not changed compared to the MSCs of the donors. MSCs from patients with acute leukemia were characterized by a decrease in SOX9 and an increase in CSF1, IL1b, and IL1bR1 expression. In MSCs of patients with myeloid leukemia, the PPARG and PDGFRA were increased. Gene expression in MSCs from DLBCL patients was significantly different from that in patients with leukemia.

Conclusion
Altered functions of the stromal microenvironment in patients indicate its role in the maintenance and proliferation of tumor cells. The findings could pave the way for new therapies that target the altered stromal niche for hematopoietic cells.
The materials supported by grants from the Russian Foundation for Basic Research project 17-00-00170 and 19-29-04023 were used.
Keyword(s): Acute leukemia, Chronic myeloid leukemia, Diffuse large B cell lymphoma, Mesenchymal stem cell


