
Contributions
Abstract: EP747
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Clinical
Background
Orva-cel is a B-cell maturation antigen (BCMA)-targeted chimeric antigen receptor (CAR) T cell therapy being evaluated in the phase 1/2 EVOLVE study (NCT03430011) in patients with relapsed/refractory multiple myeloma (RRMM) who had at least 3 prior lines of therapy. We previously reported safety and efficacy in the EVOLVE phase 1 study and established the recommended dose (RD) of orva-cel as 600 × 106 CAR+ T cells (Mailankody S, et al. J Clin Oncol. 2020;38(suppl):abstr 8504).
Aims
Cytokine release syndrome (CRS), a dominant toxicity of CAR T cell therapy, is mediated in part by interleukin-1 (IL-1). Here, we report clinical outcomes of a cohort of phase 1 patients treated prophylactically with anakinra, an IL-1 signaling inhibitor, with the objective of assessing any reduction in the incidence of grade ≥2 CRS after orva-cel treatment at the RD.
Methods
Fourteen patients were enrolled sequentially for anakinra prophylaxis and treated with orva-cel at the RD. The non–anakinra prophylaxis control group comprised the remainder of the phase 1 patients receiving orva-cel at the RD (n = 19). The median follow-up (range) was 3.0 months (1.8–6.2) for the anakinra prophylaxis group and 8.8 months (5.3–12.2) for the non–anakinra prophylaxis group. Anakinra 100 mg was administered subcutaneously the night before orva-cel infusion, 3 hours before the infusion (Day 1), and every 24 hours on Days 2–5. Dosing was increased to every 12 hours if CRS developed. CRS was graded by Lee (2014) criteria. Tocilizumab and corticosteroids were used per protocol-specified treatment management guidelines.
Results
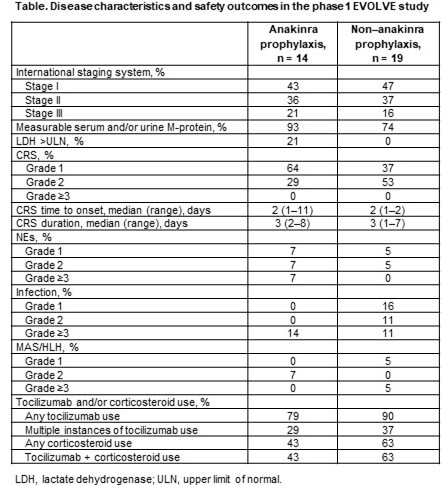
Disease characteristics and outcomes are shown in the Table. In the anakinra prophylaxis and non–anakinra prophylaxis groups, the median number of prior regimens was 6 and 5, and bridging therapy was used in 57% and 68% of patients, respectively. The total frequency of CRS was similar in the 2 groups, but with less grade 2 events in the anakinra prophylaxis patients (relative risk, 0.54 [95% CI, 0.21–1.38]). No grade ≥3 CRS was observed in either group. The incidence of neurological events (NEs), grade ≥3 infection, and macrophage activation syndrome/hemophagocytic lymphohistiocytosis (MAS/HLH) was similar. Tocilizumab and corticosteroid use was numerically lower with anakinra prophylaxis. Orva-cel expansion kinetics were similar in the 2 groups. All patients had a 2-month efficacy assessment, with an ORR of 100% in anakinra prophylaxis and 95% in non–anakinra prophylaxis patients.
Conclusion
In this nonrandomized evaluation of anakinra prophylaxis with orva-cel treatment, the incidence of grade ≥2 CRS was lower in patients receiving anakinra prophylaxis. The use of anakinra prophylaxis had no adverse effect on the incidence of NEs, infection, or MAS/HLH, nor on orva-cel expansion or disease response. These results warrant further study of anakinra prophylaxis in CAR T cell therapy.
Keyword(s): CAR-T, Multiple myeloma, Prophylaxis, Safety
Abstract: EP747
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Clinical
Background
Orva-cel is a B-cell maturation antigen (BCMA)-targeted chimeric antigen receptor (CAR) T cell therapy being evaluated in the phase 1/2 EVOLVE study (NCT03430011) in patients with relapsed/refractory multiple myeloma (RRMM) who had at least 3 prior lines of therapy. We previously reported safety and efficacy in the EVOLVE phase 1 study and established the recommended dose (RD) of orva-cel as 600 × 106 CAR+ T cells (Mailankody S, et al. J Clin Oncol. 2020;38(suppl):abstr 8504).
Aims
Cytokine release syndrome (CRS), a dominant toxicity of CAR T cell therapy, is mediated in part by interleukin-1 (IL-1). Here, we report clinical outcomes of a cohort of phase 1 patients treated prophylactically with anakinra, an IL-1 signaling inhibitor, with the objective of assessing any reduction in the incidence of grade ≥2 CRS after orva-cel treatment at the RD.
Methods
Fourteen patients were enrolled sequentially for anakinra prophylaxis and treated with orva-cel at the RD. The non–anakinra prophylaxis control group comprised the remainder of the phase 1 patients receiving orva-cel at the RD (n = 19). The median follow-up (range) was 3.0 months (1.8–6.2) for the anakinra prophylaxis group and 8.8 months (5.3–12.2) for the non–anakinra prophylaxis group. Anakinra 100 mg was administered subcutaneously the night before orva-cel infusion, 3 hours before the infusion (Day 1), and every 24 hours on Days 2–5. Dosing was increased to every 12 hours if CRS developed. CRS was graded by Lee (2014) criteria. Tocilizumab and corticosteroids were used per protocol-specified treatment management guidelines.
Results
Disease characteristics and outcomes are shown in the Table. In the anakinra prophylaxis and non–anakinra prophylaxis groups, the median number of prior regimens was 6 and 5, and bridging therapy was used in 57% and 68% of patients, respectively. The total frequency of CRS was similar in the 2 groups, but with less grade 2 events in the anakinra prophylaxis patients (relative risk, 0.54 [95% CI, 0.21–1.38]). No grade ≥3 CRS was observed in either group. The incidence of neurological events (NEs), grade ≥3 infection, and macrophage activation syndrome/hemophagocytic lymphohistiocytosis (MAS/HLH) was similar. Tocilizumab and corticosteroid use was numerically lower with anakinra prophylaxis. Orva-cel expansion kinetics were similar in the 2 groups. All patients had a 2-month efficacy assessment, with an ORR of 100% in anakinra prophylaxis and 95% in non–anakinra prophylaxis patients.

Conclusion
In this nonrandomized evaluation of anakinra prophylaxis with orva-cel treatment, the incidence of grade ≥2 CRS was lower in patients receiving anakinra prophylaxis. The use of anakinra prophylaxis had no adverse effect on the incidence of NEs, infection, or MAS/HLH, nor on orva-cel expansion or disease response. These results warrant further study of anakinra prophylaxis in CAR T cell therapy.
Keyword(s): CAR-T, Multiple myeloma, Prophylaxis, Safety


