
Contributions
Abstract: EP745
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Clinical
Background
Sickle cell disease (SCD) is a severe genetic disease for which the only cure is allogeneic hematopoietic stem cell transplant (HSCT), but this procedure requires myeloablative conditioning (usually with busulfan) to allow donor cell engraftment at the risk of chemotherapy-related toxicities, including short-term (e.g. neutropenia, veno-occlusive disease) and long-term (e.g. ovarian failure, secondary leukemia) effects. Reduced-intensity conditioning (RIC) regimens may reduce toxicities, thus expanding access to gene therapy for SCD. ARU-1801 gene therapy (autologous CD34+ hematopoietic stem cells and progenitors [HSPCs] transduced with a lentiviral vector [LV] encoding a modified γ-globinG16D gene) is being investigated with a melphalan RIC regimen (140 mg/m2). We previously reported data on 3 patients; while two had stable engraftment, one patient had a gradual decline in VCN over time, potentially linked to sub-target melphalan exposure due to rapid clearance from renal hyperfiltration (GFR = 200 mL/min/1.73m2). Melphalan plasma exposure may be a key variable to efficacy of ARU-1801 gene therapy. Melphalan clearance is dependent on renal function and SCD is often associated with renal injury. Pharmacokinetic (PK) model-based dose adjustment may lead to reduced variability and optimized melphalan exposure.
Aims
To evaluate the potential of a model-based, individualized melphalan dosing strategy for ARU-1801 in SCD.
Methods
Three patients with SCD in the ongoing Phase 1/2 MOMENTUM study received a single IV dose of melphalan 140 mg/m2 conditioning off-label prior to infusion of ARU-1801 gene therapy. PK samples were collected for 6 hours post-end of infusion (dried blood spots for first 2 subjects, plasma for third subject, assayed by direct ionization or liquid chromatography/mass spectroscopy, respectively). Blood-to-plasma ratio and hematocrit were used to convert data from blood PK samples to plasma concentration. Plasma PK parameters were determined using non-compartmental methods and the observed area under the plasma concentration-time curve (AUC) was compared to predicted values based on a population PK model of melphalan from 100 patients with multiple myeloma undergoing HSCT (Nath et al, BJCP 2010). Predicted AUCs were calculated based on melphalan dose and model-predicted plasma melphalan clearance, based on patient covariate values collected prior to melphalan infusion: fat-free-mass (FFM), hematocrit, and eGFR (based on Cystatin-C).
Results
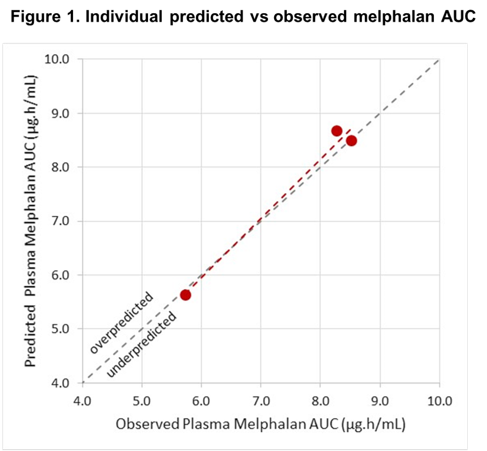
Covariate values showed large variability across the 3 patients (2 female, FFM: 38.8 to 53.0 kg, BMI: 18.7 to 28.9 kg/m2, hematocrit: 31.0 to 38.3, Cystatin-C eGFR 78 to 200 mL/min/1.73m2). Preliminary geometric mean (and coefficient of variation) plasma melphalan AUC from 3 patients with SCD was 7.39 µg.h/mL (22.4%). The observed AUC agreed well with predicted values from the PK model, with r2 = 0.98 (Figure 1). Notably, the model accurately predicted a low melphalan AUC in the patient with eGFR of 200 mL/min/1.73m2 (predicted: 5.62, observed: 5.73 µg.h/mL).
Conclusion
Model-based predictions of melphalan AUC in patients with SCD appear to be accurate across a wide range of covariate values. These data suggest individualized melphalan dosing prior to ARU-1801 infusion may be feasible; additional data will be used to refine the PK model for patients with SCD. A simple melphalan dose adjustment algorithm based on few covariate values has the potential to reduce variability and optimize melphalan exposure, which may result in improved patient outcomes after treatment with ARU-1801.
Keyword(s): Autologous hematopoietic stem cell transplantation, Gene therapy, Pharmacokinetic, Sickle cell disease
Abstract: EP745
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Clinical
Background
Sickle cell disease (SCD) is a severe genetic disease for which the only cure is allogeneic hematopoietic stem cell transplant (HSCT), but this procedure requires myeloablative conditioning (usually with busulfan) to allow donor cell engraftment at the risk of chemotherapy-related toxicities, including short-term (e.g. neutropenia, veno-occlusive disease) and long-term (e.g. ovarian failure, secondary leukemia) effects. Reduced-intensity conditioning (RIC) regimens may reduce toxicities, thus expanding access to gene therapy for SCD. ARU-1801 gene therapy (autologous CD34+ hematopoietic stem cells and progenitors [HSPCs] transduced with a lentiviral vector [LV] encoding a modified γ-globinG16D gene) is being investigated with a melphalan RIC regimen (140 mg/m2). We previously reported data on 3 patients; while two had stable engraftment, one patient had a gradual decline in VCN over time, potentially linked to sub-target melphalan exposure due to rapid clearance from renal hyperfiltration (GFR = 200 mL/min/1.73m2). Melphalan plasma exposure may be a key variable to efficacy of ARU-1801 gene therapy. Melphalan clearance is dependent on renal function and SCD is often associated with renal injury. Pharmacokinetic (PK) model-based dose adjustment may lead to reduced variability and optimized melphalan exposure.
Aims
To evaluate the potential of a model-based, individualized melphalan dosing strategy for ARU-1801 in SCD.
Methods
Three patients with SCD in the ongoing Phase 1/2 MOMENTUM study received a single IV dose of melphalan 140 mg/m2 conditioning off-label prior to infusion of ARU-1801 gene therapy. PK samples were collected for 6 hours post-end of infusion (dried blood spots for first 2 subjects, plasma for third subject, assayed by direct ionization or liquid chromatography/mass spectroscopy, respectively). Blood-to-plasma ratio and hematocrit were used to convert data from blood PK samples to plasma concentration. Plasma PK parameters were determined using non-compartmental methods and the observed area under the plasma concentration-time curve (AUC) was compared to predicted values based on a population PK model of melphalan from 100 patients with multiple myeloma undergoing HSCT (Nath et al, BJCP 2010). Predicted AUCs were calculated based on melphalan dose and model-predicted plasma melphalan clearance, based on patient covariate values collected prior to melphalan infusion: fat-free-mass (FFM), hematocrit, and eGFR (based on Cystatin-C).
Results
Covariate values showed large variability across the 3 patients (2 female, FFM: 38.8 to 53.0 kg, BMI: 18.7 to 28.9 kg/m2, hematocrit: 31.0 to 38.3, Cystatin-C eGFR 78 to 200 mL/min/1.73m2). Preliminary geometric mean (and coefficient of variation) plasma melphalan AUC from 3 patients with SCD was 7.39 µg.h/mL (22.4%). The observed AUC agreed well with predicted values from the PK model, with r2 = 0.98 (Figure 1). Notably, the model accurately predicted a low melphalan AUC in the patient with eGFR of 200 mL/min/1.73m2 (predicted: 5.62, observed: 5.73 µg.h/mL).

Conclusion
Model-based predictions of melphalan AUC in patients with SCD appear to be accurate across a wide range of covariate values. These data suggest individualized melphalan dosing prior to ARU-1801 infusion may be feasible; additional data will be used to refine the PK model for patients with SCD. A simple melphalan dose adjustment algorithm based on few covariate values has the potential to reduce variability and optimize melphalan exposure, which may result in improved patient outcomes after treatment with ARU-1801.
Keyword(s): Autologous hematopoietic stem cell transplantation, Gene therapy, Pharmacokinetic, Sickle cell disease


