
Contributions
Abstract: EP738
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Clinical
Background
T-cell depletion, especially alemtuzumab, reduces the risk of graft-versus-host disease (GVHD) following allogeneic hematopoietic stem cell transplantation (HSCT) using unrelated donor peripheral stem cell graft (PBSC-matched/mismatched), but has been associated with an increased frequency of mixed t cell chimerism (MC) and subsequent relapse. In adult patients receiving alemtuzumab and having MC (T cell), early pre-emptive donor lymphocyte infusion (pDLI) has been safe and effective in reducing relapses, without increasing risk of graft vs host disease (GVHD). Among responders in paediatric cohort having lineage specific MC(CD34/33), pDLI could achieve outcomes nearly similar to patients having complete chimerism (CC). However, many patients having MC actually fail to receive timely pDLI, and their outcomes remain particularly dismal, which is not considered. Thus, we compared long term outcomes of patients with T cell MC (including patients not receiving pDLI) to patients having complete chimerism CC
Aims
Thus, we compared long term outcomes of patients with T cell MC (including patients not receiving pDLI) to patients having complete chimerism CC for overall survival, disease free survival and other outcomes
Methods
One-hundred six adult patients with CD3 MC after day 60, in patients undergoing HSCT for acute leukaemia/myelodysplastic syndrome (MDS) from an unrelated donor (UD), using alemtuzumab and predominantly PBSC grafts, between 2007-2016, were compared with 111 patients having CC. CD33 chimerism was >98%. In patients with MC intention was to start pDLI (by day 100) after rapid withdrawal of immunosuppressants (chimerism <50%).
Results
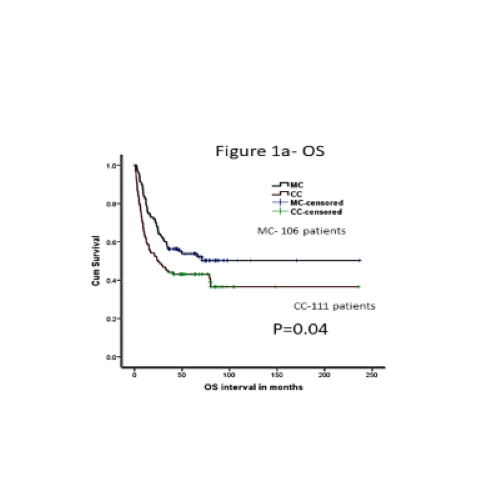
Both groups were comparable for age, mismatches, graft source, and disease risk. MC group had more patients receiving reduced intensity (RIC) regimen (62% vs 52% in CC, p=0.15). The median follow-up was 33 (0.6-150 months). Fifty- three (56%) patients received pDLI. The median dose of pDLI was 1 x106/kg and median time to pDLI was 5 months from transplantation. Out of 56 patients, 36 patients (67%) had a response (RR) (85% achieving CC) and 17 (33%) patients had no response (NR). Fifty patients (44%) did not receive any pDLI (ND). pDLI was well tolerated with no difference in GVHD (p=0.65), infections (p=0.37) or NRM (P=0.37) between pDLI and ND. Overall survival (OS) was significantly better in MC group as compared to CC (52.4% vs 42%, p=0.04), mainly due to reduction in non- relapse mortality NRM (14% vs 26%, p=0.05) and all grade acute and chronic GVHD (38% vs 68%, p=0.001, and 37% vs 51%, p=0.025). Relapses and disease-free survival were comparable between the two groups (32% vs 38%, p=0.99 and 38.5% vs 45%, p=0.12, for CC and MC, respectively). After multivariate analysis, MC still had significantly better OS (p=0.02, HR-1.53, CI-1.0-2.2) and NRM (p=0.02, HR-2.44, CI-1.3-5.2, ref MC). Within MC group, response to pDLI was the only significant factor predicting OS, DFS and relapses with NR and ND having unfavourable outcomes as compared to RR (p=0.0001, HR=5.45, and p=0.001, HR-5.95, respectively).
Conclusion
In this large single centre study, we have shown that T cell MC in patients undergoing UD allografts with alemtuzumab is no longer an adverse prognostic factor, with timely initiation of pre-emptive pDLI, and their OS is in fact better than CC, mainly due to reduction in NRM and early onset aggressive GVHD. This strategy is safe and well tolerated. Response to pDLI is the main independent predictor of overall outcomes in patients with MC.
Keyword(s): Acute leukemia, Allogeneic bone marrow transplant, Donor lymphocyte infusion, Prophylaxis
Abstract: EP738
Type: E-Poster Presentation
Session title: Gene therapy, cellular immunotherapy and vaccination - Clinical
Background
T-cell depletion, especially alemtuzumab, reduces the risk of graft-versus-host disease (GVHD) following allogeneic hematopoietic stem cell transplantation (HSCT) using unrelated donor peripheral stem cell graft (PBSC-matched/mismatched), but has been associated with an increased frequency of mixed t cell chimerism (MC) and subsequent relapse. In adult patients receiving alemtuzumab and having MC (T cell), early pre-emptive donor lymphocyte infusion (pDLI) has been safe and effective in reducing relapses, without increasing risk of graft vs host disease (GVHD). Among responders in paediatric cohort having lineage specific MC(CD34/33), pDLI could achieve outcomes nearly similar to patients having complete chimerism (CC). However, many patients having MC actually fail to receive timely pDLI, and their outcomes remain particularly dismal, which is not considered. Thus, we compared long term outcomes of patients with T cell MC (including patients not receiving pDLI) to patients having complete chimerism CC
Aims
Thus, we compared long term outcomes of patients with T cell MC (including patients not receiving pDLI) to patients having complete chimerism CC for overall survival, disease free survival and other outcomes
Methods
One-hundred six adult patients with CD3 MC after day 60, in patients undergoing HSCT for acute leukaemia/myelodysplastic syndrome (MDS) from an unrelated donor (UD), using alemtuzumab and predominantly PBSC grafts, between 2007-2016, were compared with 111 patients having CC. CD33 chimerism was >98%. In patients with MC intention was to start pDLI (by day 100) after rapid withdrawal of immunosuppressants (chimerism <50%).
Results
Both groups were comparable for age, mismatches, graft source, and disease risk. MC group had more patients receiving reduced intensity (RIC) regimen (62% vs 52% in CC, p=0.15). The median follow-up was 33 (0.6-150 months). Fifty- three (56%) patients received pDLI. The median dose of pDLI was 1 x106/kg and median time to pDLI was 5 months from transplantation. Out of 56 patients, 36 patients (67%) had a response (RR) (85% achieving CC) and 17 (33%) patients had no response (NR). Fifty patients (44%) did not receive any pDLI (ND). pDLI was well tolerated with no difference in GVHD (p=0.65), infections (p=0.37) or NRM (P=0.37) between pDLI and ND. Overall survival (OS) was significantly better in MC group as compared to CC (52.4% vs 42%, p=0.04), mainly due to reduction in non- relapse mortality NRM (14% vs 26%, p=0.05) and all grade acute and chronic GVHD (38% vs 68%, p=0.001, and 37% vs 51%, p=0.025). Relapses and disease-free survival were comparable between the two groups (32% vs 38%, p=0.99 and 38.5% vs 45%, p=0.12, for CC and MC, respectively). After multivariate analysis, MC still had significantly better OS (p=0.02, HR-1.53, CI-1.0-2.2) and NRM (p=0.02, HR-2.44, CI-1.3-5.2, ref MC). Within MC group, response to pDLI was the only significant factor predicting OS, DFS and relapses with NR and ND having unfavourable outcomes as compared to RR (p=0.0001, HR=5.45, and p=0.001, HR-5.95, respectively).

Conclusion
In this large single centre study, we have shown that T cell MC in patients undergoing UD allografts with alemtuzumab is no longer an adverse prognostic factor, with timely initiation of pre-emptive pDLI, and their OS is in fact better than CC, mainly due to reduction in NRM and early onset aggressive GVHD. This strategy is safe and well tolerated. Response to pDLI is the main independent predictor of overall outcomes in patients with MC.
Keyword(s): Acute leukemia, Allogeneic bone marrow transplant, Donor lymphocyte infusion, Prophylaxis


